|
Cell nucleus activity during post-embryonic development of Apis mellifera L. (Hymenoptera: Apidae). Intranuclear acid phosphatase
ABSTRACT. We report nuclear acid phosphatase activity in the somatic (intra-ovariolar and stromatic) and germ cells of differentiating honey bee worker ovaries, as well as in the midgut cells of metamorphosing bees. There was heterogeneity in the intensity and distribution of electron dense deposits of lead phosphate, indicative of acid phosphatase activity in the nuclei of these tissues, during different phases of post-embryonic bee development. This heterogeneity was interpreted as a variation of the nuclear functional state, related to the cell functions in these tissues. Key words: Intranuclear acid phosphatase activity, Apis mellifera, Post-embryonic development, Honey bee
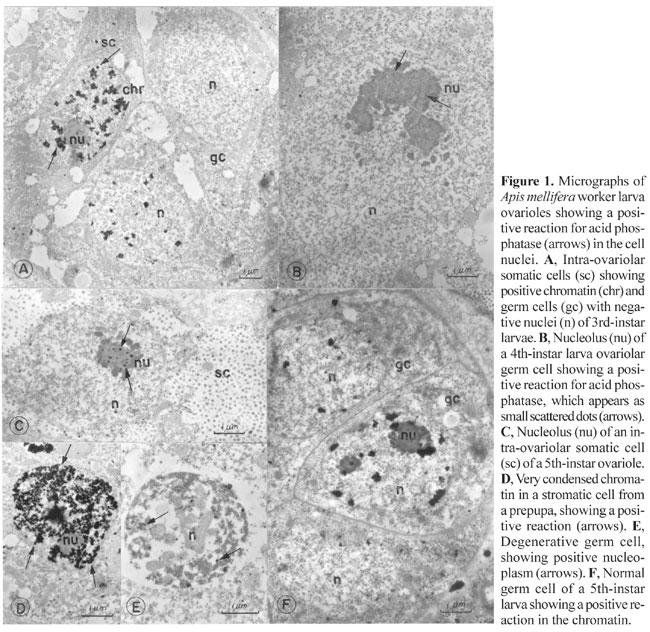
INTRODUCTION There have been several reports of acid phosphatase activity in cell nuclei, but its role in this organelle remains unknown. Acid phosphatase activity was found by Love et al. (1969), Soriano and Love (1971), Vorbrodt (1974) and Buchwalow and Unger (1977) in animal cell nuclei and by DeJong et al. (1967), Walbot (1971), Van de Walle et al. (1976), Risueño et al. (1976) and Deltour et al. (1981) in plant cell nuclei. The apparent presence of acid phosphatase in the nucleus has been variously explained as inorganic phosphate accumulation, as an artifact due to non-specific lead deposits, or to diffusion of reaction products from the cytoplasm to the nucleus during staining (Barka and Anderson, 1962; Washitam and Sato, 1976). However, there are convincing arguments for real intranuclear acid phosphatase activity under certain conditions. Methods that eliminate artifactual lead deposits diminish, but do not eliminate, nuclear labeling (Barka and Anderson, 1962; Poux, 1967, 1970; Pfeifer et al., 1974). However, positive reactions obtained with isolated nuclei (Deltour et al., 1981) and the elimination of inorganic phosphate by glutaraldehyde fixation (Tandler and Solari, 1969) eliminate the possibility of reaction product diffusion and inorganic phosphate involvement, respectively. Deltour et al. (1981) noticed differences in lead phosphate deposits between nuclei of quiescent and germinative embryos of Zea mays, and in cells before and after thermal shock. These results, along with those of Van de Walle et al. (1976) suggested a relation between nuclear transcription and acid phosphatase activity. This view is supported by the fact that transcription inhibition is accompanied by suppression of acid phosphatase activity in the cell nuclei of some plants (Walbot, 1971; Risueño et al., 1976). In the more recent literature, this subject has only been treated by Oliveira (1997). Nevertheless, nuclear components frequently are acid phosphatase positive; this varies with the cell type or cell phase. We studied the conditions under which nuclear acid phosphatase activity occurs in the ovaries and midgut of immature bees, to determine how this enzyme is related to the functional state of the nucleus. MATERIAL AND METHODS Ovaries and midgut cells from Apis mellifera L. (Hymenoptera, Apidae) were studied with transmission electron microscopy using b-glycerophosphate and p-nitrophenyl phosphate as substrates to detect acid phosphatase activity. The worker ovaries were examined during larval development and at the beginning of pupation, and the midgut was studied during metamorphosis (pupation). The progression of pupal development was monitored by examining eye and body pigmentation, starting with pre-pupae and following a sequence of darkening of the eyes and body. The material, fixed in 2.5% 0.1 M sodium cacodylate buffer, pH 7.2, for 1 h, was incubated in a medium containing the substrate and post-fixed in 1% osmium tetroxide in the same buffer, for 2 h. Some specimens were incubated in a medium devoid of substrate or in a medium containing substrate plus sodium fluoride, an enzyme inhibitor. The thin sections were first examined without contrast, although the photos were taken with uranyl acetate- and lead citrate-contrasted grids in order to obtain better visualization. RESULTS Ovary Two types of cells are present in the ovary, the somatic and the germ cells. Germ cells were found only inside the ovarioles and somatic cells were present both in ovarioles and stromatic tissue, which in immature phases fills up the spaces among the ovarioles. Electron-dense deposits of lead phosphate, indicative of acid phosphatase activity, were detected in some nuclei of somatic and germ cells in the presence of substrate. The intensity and location of the labeling changed according to the developmental phase. A positive reaction was seen in ovary cells of 3rd-, 4th- and 5th-stage worker larvae, i.e., from the beginning of worker-queen ovary divergence, to the end of the larval stage, or the end of ovary differentiation (Figure 1).
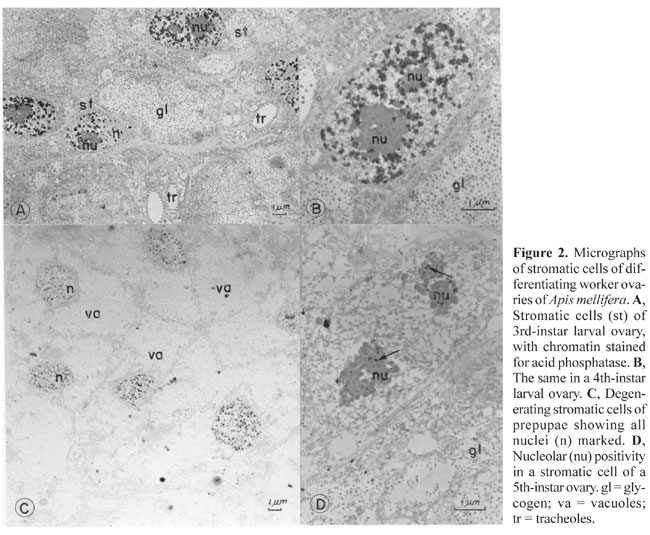
Chromatin (Figure 1A,D) and nucleoli (Figure 1C) were stained in the intra-ovariolar somatic cells of 3rd- and 4th-instar larvae. The cells with positive nuclei were apparently normal, active cells, with rounded nuclei, disperse chromatin and prominent nucleoli. The cytoplasm showed glycogen deposits, as the main feature, and no lead staining. Some cells had unstained nuclei (Figure 1A). Chromatin and nucleolus staining were never seen in the same nucleus (Figure 1A,B,C). In the germ cells, a positive reaction was found in the nucleoli of 4th-instar larvae (Figure 1B) and in the nucleoplasm of condensed nuclei in 3rd-, 4th- and 5th-instar larvae (Figure 1E). The cells with condensed nuclei exhibited a positive reaction for acid phosphatase, as well as in cytoplasmic vacuoles, as characteristic of damage. The other cells with labeled nucleoli had a normal appearance. The nuclei had very disperse chromatin, and a large nucleolus. The labeling of the nucleolus appeared as small dots in germ cells and as small clumps within the inner compact mass of the nucleolus in somatic cells. The peripheral thread-like part of this organelle was not stained (Figure 1B,C). A positive reaction in the nuclear chromatin of germ cells (Figure 1F) was seen in the 5th-instar larvae, though it appeared less intense than in somatic intra-ovariolar cells (Figure 1A). The positivity for acid phosphatase in the stromatic cells was stronger (Figure 1D) than in intra-ovariolar somatic cells (Figure 1A). Here, the intensity of the reaction was also different from cell to cell, and some nuclei did not stain (Figure 2). A positive reaction was detected in the chromatin of stromatic cell nuclei of 3rd- and 4th-instar larvae (Figure 2A,B), while in 5th-instar
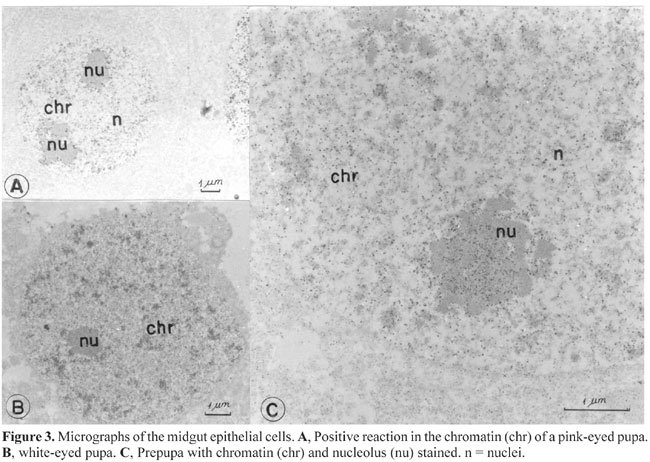
only the nucleoli (Figure 2D) were labeled. The positive nuclei of pre-pupal stromatic cells had labeled chromatin and nucleoli (Figure 2C). All the cells of 3rd- and 4th-instar ovarioles with positive nuclei appeared normal, while those of pre-pupae had a modified shape; the nuclei were smaller and surrounded by cytoplasm containing very large and apparently empty vacuoles (Figure 2C). Midgut In the midgut, a positive reaction for acid phosphatase appeared in some epithelial cells of white, pink and brown-eyed pupae (Figure 3). Only the chromatin was positive in white-eyed pupae (Figure 3A), while in pink and brown-eyed pupae both chromatin and the nucleolus were stained (Figure 3B,C). The nucleolus stained more intensely than the chromatin. The chromatin in the brown-eyed pupae (Figure 3B) seemed to be more condensed and less stained than in white-eyed pupae (Figure 3C). All cells with stained nuclei, as well as the labeled nuclei, seemed normal.
The absence of substrate and the use of phosphatase inhibitor eliminated lead phosphate deposits. DISCUSSION Acid phosphatase activity in the nuclei was reported more than 30 years ago. Siebert (1966) found activity in rat liver cells using biochemical techniques. Later, other authors demonstrated acid phosphatase activity using cytochemical studies (Soriano and Love, 1971; Miyayama et al., 1975; Sanchez-Pina et al. 1978; Deltour et al, 1981; Azeredo-Oliveira, 1982; Oliveira, 1997). There was no lead deposition in the control preparations of bee tissues incubated with lead nitrate, both without substrate and with acid phosphatase activity inhibitors; therefore it can be assumed that nuclear staining indicates acid phosphatase activity. This is in accordance with Deltour et al. (1981) who stated that staining of this enzyme was not artifactual, because it could be stained and demonstrated biochemically in isolated nuclei. Activity sites for acid phosphatase other than the nucleus, including cytoplasmic vacuoles, were detected in these tissues. Chromatin, nucleoli and nucleoplasm, were not simultaneously positive. This probably means that the lead staining corresponds to direct visualization of acid phosphatase activity. There was considerably heterogeneity in the presence and distribution of acid phosphatase among the bee cell nuclei, independent of their nature (somatic or germinative) or origin (ovary or midgut). This may reflect differences in the functional state of the nuclei, according to Deltour et al. (1981) who found strong acid phosphatase activity in highly (metabolically) active root-cell nuclei during plant seed germination. They also found various sites of enzymatic activity in the nuclei. Azeredo-Oliveira (1982) and Oliveira (1997) also found staining for acid phosphatase in insect cell chromatin and nucleoli. Oliveira used Ag impregnation of the nucleolus organizer region (AgNOR) to identify the nucleolus as the structure showing lead phosphate precipitate. He also suggested that acid phosphatase was active during rRNA transcription in the fibrillar center of the nucleolus. Based on the foregoing evidence, we postulate a relationship between the intensity of the reaction and the level of transcription in bee-cell chromatin. This may also be true for nucleoli, since the staining inside the nucleolar mass and the distribution of the chromosome nucleolar organizer chromatin tend to coincide. As staining of the nuclear structures occurs in different tissues, and in the same tissue at different sites and with different intensities, we can make correlations between acid phosphatase activity and cell function. The lack of staining in some intra-ovariolar cells in 3rd- and 4th-instar larvae may mean that they are germ cells with a low transcription rate at this developmental stage. An increase in the transcription rate in these cells would be expected only during the later developmental phases, especially in the nucleolus. All the acid phosphatase positive cells had a normal morphology except some of the germ cells, which had condensed nuclei being reabsorbed, and prepupal stromatic cells. The enzymatic activity registered at this phase of development may be due to a hydrolytic function of acid phosphatase, since most of the larval tissue is degenerating in prepupae (Cruz-Landim and Silva de Moraes, 2000). This may explain the lead phosphate deposits in the nucleoplasm, due to a hydrolytic function for this enzyme, probably affecting the protein of the nuclear skeleton. Lead deposits in nucleoli during the prepupal phase may on the other hand indicate rRNA production for the synthesis of new proteins related to the cell death process (Bowen et al., 1993). Acid phosphatase reaction heterogeneity was also found in the nuclei of stromatic cells. A high metabolic activity in stromatic cells was not expected, but some of them may be involved in the production of hormone-like substances that regulate ovariole differentiation and germ cell proliferation. In prepupae, acid phosphatase staining of the nuclei was less common, but when it occurred the staining was more intense, which may reflect the initiation of involution of this tissue. The presence of lead deposits in intact nuclei of normal digestive tract cells, more intense in the oldest pupae (brown eyes) and in the nucleoli than in chromatin, may be interpreted as preparation for enzyme synthesis, which will be necessary in the adult bees. There are reports of involvement of alkaline phosphatase in the control of the cellular cycle (Murray and Kirschner, 1991) and in the hydrolysis of o-phosphotyrosine during the prepupal phase in insects (Harper and Armstrong, 1972). Acid phosphatase could have similar cell-signaling functions, through the dephosphorylation of specific nuclear components, which would mean that acid phosphatase controls gene expression. ACKNOWLEDGMENTS The authors are grateful to CNPq and FAPESP for financial support. REFERENCES Azeredo-Oliveira, M.T.V. (1982). Estudo citoenzimológico em túbulos de Malpighi de Triatoma infestans Klug. Master’s thesis, UNICAMP, Campinas, SP, Brazil. Barka, T. and Anderson, P.J. (1962). Histochemical methods for acid phosphatase using hexazonium pararosanilin as coupler. J. Histochem. Cytochem. 10: 741-753. Bowen, I.D., Morgan, S.M. and Mullarkey, K. (1993). Cell death in the salivary glands of metamorphosing Calliphora vomitoria. Cell Biol. Int. Rep. 17: 13-33. Buchwalow, I.B. and Unger, E. (1977). Enzyme activity of nuclear ribonucleoproteins. Exp. Cell Res. 106: 139-152. Cruz-Landim, C. and Silva de Moraes, R.L.M. (2000). Morte celular programada em abelhas como uma forma de redirecionar a morfologia e fisiologia adaptativa. Ed. Costa, Rio Claro, SP, Brazil, pp. 47. DeJong, D.W., Olson, A.C. and Jansen, E.F. (1967). Glutaraldehyde activation of nuclear acid phosphatase in cultured plant cells. Science 155: 1672-1674. Deltour, R., Fransolet, S. and Loppes, R. (1981). Inorganic phosphate accumulation and phosphatase activity in the nucleus of maize embryo root cells. J. Cell Sci. 47: 77-89. Harper, R.A. and Armstrong, F.B. (1972). Alkaline phosphatase of Drosophila melanogaster. 1. Partial purification and characterization. Biochem. Genet. 6: 75-82. Love, R., Studzinski, G.P. and Walsh, R.J. (1969). Nuclear nucleolinar and cytoplasmatic acid phosphatases in cultured mammalian cells. Exp. Cell Res. 58: 62-72. Miyayama, H., Salomon, R., Sasaki, M., Liu, C.-W. and Fishman, W.H. (1975). Demonstration of lysosomal and extralysosomal sites for acid phosphatase in mouse kidney tubule cells with p-nitrophenyl phosphate lead-salt technique. J. Histochem. Cytochem. 23: 439-451. Murray, A.W. and Kirschner, M.W. (1991). What controls the cell cycle. Sci. Am. 264: 57-63. Oliveira, A.P.M.L. (1997). Estudo citoquímico em glândulas salivares de triatomídeos do gênero Rhodnius. Master’s thesis, UNESP, São José do Rio Preto, SP, Brazil. Pfeifer, V., Pehlmann, E. and Witschel, H. (1974). Kinetics of the accumulation of lead phosphate in acid phosphatase staining. In: Electron Microscopy and Cytochemistry (Daems, W.W., Molenaae, I. and van Duyn, P., eds.). North Holland Publishing, Amsterdam and London, pp. 25-28. Poux, N. (1967). Localisation d’activités enzymatiques dans les cellules du méristème radiculaire de Cucumis satinis L. I. Activités phosphotasiques neutres dans les cellules du protoderme. J. Microsc. 6: 1043-1058. Poux, N. (1970). Localisation d’activités enzymatiques dans le méristème radiculaire de Cucumis sativus L. III. Activité phosphatasique acide. J. Microsc. 9: 407-434. Risueño, M.C., Moreno Díaz de La Espina, S., Fernandez-Gomez, M.E. and Giménez-Martin, G. (1976). Ultrastructural study of nucleolar material during plant mitosis in the presence of inhibitors of RNA synthesis. J. Microsc. Biol. Cell. 26: 5-18. Sanchez-Pina, A., Rodriguez-Garcia, M.I., Risueno, M.C. and Fernandez-Gomez, M.E. (1978). Localization of the acid phosphatasic activity in plant cell nucleoli. Rev. Microsc. Eletron. 5: 228-232. Siebert, G. (1966). Nucleolar enzymes of isolated rat liver nucleoli. Natl. Cancer Inst. Monogr. 23: 285-293. Soriano, R.Z. and Love, R. (1971). Electron microscopic demonstration of acid phosphatase in nucleoli and nucleoplasm. Exp. Cell Res. 65: 467-470. Tandler, C.J. and Solari, A.J. (1969). Nucleolar orthophosphate ions. Electron microscope and diffraction studies. J.Cell Biol. 41: 91-108. Van de Walle, C., Bernier, G., Deltour, R. and Bronchart, R. (1976). Sequence of reactivation of ribonucleic acid synthesis during early germination of the maize embryo. Plant Physiol. 157: 632-639. Vorbrodt, A. (1974). Cytochemistry of nuclear enzymes. In: The Cell Nucleus (Busch, H., ed.). Academic Press, New York and London, pp. 344-399. Walbot, V. (1971). RNA metabolism during embryo development and germination of Phaseolus vulgaris. Dev. Biol. 26: 369-379. Washitam, I. and Sato, S. (1976). On the reliability of the lead salt precipitation method of acid phosphatase localization in plant cells. Protoplasma 89: 157-170. |