|
A model system for testing gene vectors using murine tumor cells on the chorioallantoic membrane of the chick embryo
ABSTRACT. We developed a model system for testing gene vectors, based on the growth of murine tumors on the chorioallantoic membrane (CAM) of embryonic chickens. The ability of selected murine cells to grow on the CAM was rated according to the following criteria: i) formation of tumor masses; ii) metastasis formation; iii) reproducibility; iv) yield, indicated as the number of embryos surviving to assessment time with visible tumors on the CAM; v) maintainability of the cell, both in the original host and the embryonic chick, or “shuttle maintainability”; vi) detection by the naked eye, and vii) cost/benefit relation. The murine melanoma cell lineage, B16F10, which efficiently forms distinct, pigmented tumor masses and metastases on the CAM, performed better in this model than the murine B61 cell line. In vitro transduction of B16F10 cells with a recombinant adenovirus carrying a construct of the E. coli LacZ gene followed by inoculation onto the CAM resulted in b-galactosidase expression in the tumor mass growing on the CAM. This model is potentially applicable to preclinical evaluation of gene vectors, especially for gene therapy of cancer. Key words: Chorioallantoic membrane, Gene vector screening, Cancer gene therapy, Animal models
INTRODUCTION A gene vector must go through an extensive battery of tests, including validation of safety, specific activity and manufacturing, before it can be approved for clinical use. This process is very expensive and laborious, and it would be useful to have efficient and inexpensive methods for preclinical testing of vectors, with experimental conditions that are as similar as possible to the real in vivo situation. Transplantation of heterologous cells and tissues to the embryonic chick (Karnofsky et al., 1952) and, particularly, to the chorioallantoic membrane (CAM) (Zwilling, 1959; Hamburger, 1960; Norpoth and Rauen, 1968; Ausprunk et al., 1974, 1975; Knighton et al., 1977; Armstrong et al., 1982; Chambers et al., 1982, 1992) is an established model system to evaluate many different parameters of tumor growth (Leighton, 1964; Knighton et al., 1977; Chambers et al., 1982, 1992) and antineoplastic drug screening (Brooks et al., 1994; Stan et al., 1999). The CAM model system conveniently and inexpensively reproduces many of the characteristics of tumors in vivo, such as tumor mass formation, angiogenesis or neovascularization, infiltrative growth and metastasization. This model presents a series of advantages over conventional animal models, including simplicity, low cost, and natural immunodeficiency of the chick embryo and the CAM which makes them good hosts for a great variety of cells and tissues (Karnofsky et al., 1952; Leighton, 1964).
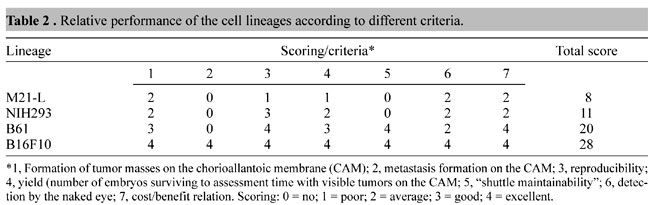
We rated the ability of murine cells (Table 1) to grow on the CAM according to the following criteria i) formation of tumor masses; ii) metastasis formation; iii) reproducibility; iv) yield,determined as the number of embryos surviving until assessment time with visible tumors on the CAM; v) maintainability of the cells both in the original host and the embryonic chick, or “shuttle maintainability”; vi) detection by the naked eye, and vii) cost/benefit relation. The murine melanoma B16F10 line yielded the best results for all criteria, followed by transformed murine fibroblast B61 line (Table 2). To test this model system for evaluating gene vectors with murine tumor cells on the CAM, we transduced B16F10 cells with a recombinant adenoviral vector carrying a Lac-Z construct and detected the expression of b-galactosidase in the tumor mass in ovo.
MATERIAL AND METHODS Fertilized hen eggs Fertilized chicken eggs were obtained from a local hatchery and were incubated in a forced-air incubator (Premium Ecológica Ltda., Belo Horizonte, MG, Brazil) with an automatic turner, at 37ºC and ~80% relative humidity until incubation day 10. Viability of the embryos was assessed daily by candling. After introduction of the tumor cells, the eggs were incubated, without turning, under the same conditions, until incubation day 17, at which time they were prepared for assessment as below. Cell culture Cells were maintained in culture under appropriate conditions for each cell type (Table 1), and were subcultured routinely by trypsinization (0.01% Trypsin, Life Sciences, USA). The following murine cell lineages were used: B61, which are murine fibroblasts transformed with a mutated ras, and murine melanoma B16F10 (Fidler, 1975). In some experiments two human cell lines, NIH293 (fetal kidney transformed by copies of the adenoviral E1 gene) and M21-L (melanoma cell lineage), were used. Cell numbers were determined with a Neubauer chamber. Cells were taken from frozen stocks at intervals of no more than three months. Cells to be inoculated on the CAMs were detached from the culture flasks by trypsinization, followed by trypsin inactivation with RPMI medium (Sigma, USA) containing 10% fetal calf serum (FCS, Life Sciences, USA). The cell suspension was transferred to microcentrifuge tubes and spun at 1500 rpm for 5 min. The supernatant was replaced with serum-free RPMI. The cell pellet (20-40 ml or 105-106 cells) was resuspended with a micropipet and applied onto the chorioallantoic epithelium (CE) of embryonic chicks, prepared as below. To grow tumors in vivo, 0.2 ml of B61 and B16F10 cells was inoculated subcutaneously in syngeneic receptors, BALB/c and C57BL mice, respectively. Fifteen days after inoculation, the tumors were extirpated, washed in sterile saline and dissociated with a 5-ml sterile syringe in serum-free RPMI supplemented with antibiotics. The suspension of dissociated cells was transferred to microcentrifuge tubes and spun as above. The cell pellet was resuspended and applied onto the CE, as above, or back into the syngeneic host, for in vivo maintenance of the cell lineage. Cell inoculation onto the chorioallantoic membrane To apply cells onto the CAM of 10-day chick embryos, a 2-cm diameter wide window was opened aseptically in the flat pole of the eggshell with an electric drill (Dremel® moto-tool, Emerson Electric Co., Racine, WI, USA) equipped with a 0.3-mm thick x 22-mm diameter Carborundum disc. The shell window was removed with forceps. The exposed surface of the dermic sheet on the floor of the air sac was wetted with a few drops of sterile PBS, then carefully punctured with fine sterile watchmaker’s forceps and removed to show the underlying CAM. Care was taken at this point to avoid any injury to the highly vascularized CAM. A small portion of the CAM was then gently traumatized by laying a 1-cm wide strip of ether-extracted lens tissue on the surface of the CE and then removing it immediately (Armstrong et al., 1982). This technique of gentle traumatization of the CAM greatly increased the ability of all cell lineages to form visible tumor masses on the CAM (data not shown). The inoculum was applied onto the small patch of traumatized CAM. Following inoculation, the window was closed with sterile, 22-mm diameter glass cover slip (Fisher Scientific, USA) sealed in place with heat-melted glue applied with an electric pistol. The embryo was returned to the incubator in an upright position, without turning, and remained there for seven additional days, with daily monitoring. At the time of harvest (~incubation day 17), the embryo was killed by hypothermia. The eggshell was carefully broken at one pole and the egg was fixed in toto in PBS-buffered 10% formalin, for 4-7 days. The CAM was then dissected out with scissors, photographed by transillumination in a Stemi SV11 stereomicroscope (Zeiss, Germany), rolled in a paper filter disc of the same size kept rolled by a knotted thread of human hair, embedded in paraffin and sliced in a microtome. In some experiments, the CAM was dissected out at the time of harvest, then rapidly fixed in formalin and processed for the b-galactosidase expression assay as below. Histological preparations were stained by hematoxylin-eosin (HE). Recombinant adenovirus The replication-deficient recombinant adenovirus, Ad-RSV-nlsLacZ, used in this study is described elsewhere (Graham and Prevec, 1991). It contains the Escherichia coli LacZ gene, encoding b-galactosidase fused to the nuclear localization signal of SV40. The expression of the cassette is driven by the Rous sarcoma virus long terminal repeat. Adenoviruses were propagated in human embryonic kidney cell line NIH293 (ATCC collection), as described previously (Graham and Prevec, 1991). Supernatants of NIH-293 cultures transduced with the adenovirus were used without further processing to transduce cells to be inoculated onto the CE/CAM. Assay of b-galactosidase activity To evaluate gene transfer and expression after administration of the adenoviral vector, the CAM was carefully dissected out at harvest; placed in a six-well plate (Corning-Costar, USA), washed twice in PBS, incubated in fixing solution [4% formaldehyde in PBS, plus 5 mM EGTA and 0.02% Nonidet-P40 (Sigma, USA)], at room temperature, for 10 min; washed three times with PBS and then stained (12 h, 37oC) by immersion in the reaction mixture, prepared as follows (Rotman, 1961, with modifications): 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 1 mg/ml X-Gal stain [5-bromo-4-chloro-3-indolyl-ß-D-galactopyranoside (Sigma, USA) dissolved in N,N-dymethylformamide (Sigma) at 20 mg/ml, and stored at -20oC prior to dilution into the reaction mixture], in PBS. After incubation in the X-Gal reaction mixture, the CAM was dehydrated in ascending ethanol concentrations and photographed by transillumination in a Stemi SV11 stereomicroscope (Zeiss, Germany). Cells were considered as positive for the expression of the lacZ product if they exhibited the characteristic perinuclear X-Gal blue color. RESULTS Lineage B16F10 grows in cell culture, in vivo and in ovo, in a much better and convenient way than the B61 lineage tested here (Figures 1-4). It produced rapidly growing, highly vascularized tumor masses in the syngeneic mouse (Figure 1), and numerous distinct metastases in the CAM (Figures 2 and 4). It also grew on the CAM from dissociated explants of the primary mouse tumor (Figure 4D). Compared to the B61 cell lineage, B16F10 has a more undifferentiated and invasive behavior (Figure 5).
DISCUSSION AND CONCLUSIONS To date, cancer is the most important target of gene therapy, both in number of trials and number of patients (Gene Therapy Clinical Trials Update - February 1, 2001). There is a number of obstacles to cancer gene therapy, ranging from natural barriers in the patient’s own body, such as the immune system, to the efficiency, targetability and tissue specificity of the gene vector being used (Dani, 1999). The results obtained with the inoculation of transduced B16F10 cells onto the chorioallantoic membrane of the embryonic chick open possibilities to test vectors on a large scale and at low cost in a model quite similar to the in vivo situation. These cells are able to grow in vitro, in vivo (in its syngeneic host) and in ovo, to form tumor masses and metastases in vivo and in ovo, with high reproducibility and yield. This lineage has the additional benefits of being maintained in vivo, thus reducing the costs of its in vitro maintenance, and is detectable by the naked eye, due to its distinctive pigmentation. The b-galactosidase gene transfer and expression assay can potentially be used for screening new genes and gene/drug combinations for antineoplastic, antiangiogenic, anti-infiltrative and anti-metastasizing activity. ACKNOWLEDGMENTS Research supported by FAPESP grant No. 97/06229-5. Cell lineages were a generous gift from Luiz Travassos (EPM/UNIFESP, Brazil), David A. Cheresh/Tanya R. Gresham (The Scripps Research Institute, USA), Mari C. Sogayar (IQ/USP, Brazil) and Roger Chammas (USP, Brazil). Ad-RSV-nlsLacZ recombinant adenovirus was kindly donated by Michel Perricaudet. We thank Alexandru C. Stan for valuable suggestions and Fábio V. Valeri for help with HE-stained histological preparations. REFERENCES Armstrong, P.B., Quigley, J.P. and Sidebottom, E. (1982). Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res 42: 1826-1837. Ausprunk, D.H., Knighton, D.R. and Folkman, J. (1974). Differentiation of vascular endothelium in chick chorioallantois: a structural and autoradiographic study. Dev. Biol. 38: 237-248. Ausprunk, D.H., Knighton, D.R. and Folkman, J. (1975). Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Role of host and preexisting graft blood vessels. Am. J. Pathol. 79: 597-628. Brooks, P.C., Montgomery, A.M., Rosenfeld, M., Reisfeld, R.A., Hu, T., Klier, G. and Cheresh, D.A. (1994). Integrin avb3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79: 1157-1164. Chambers, A.F., Shafir, R. and Ling, V. (1982). A model system for studying metastasis using the embryonic chick. Cancer Res. 42: 4018-4025. Chambers, A.F., Schmidt, E.E., MacDonald, I.C., Morris, V.L. and Groom, A.C. (1992). Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital videomicroscopy. J. Natl. Cancer Inst. 84: 797-803. Dani, S.U. (1999). The challenge of vector development in gene therapy. Braz. J. Med. Biol. Res. 32: 133-145. Fidler, I.J. (1975). Biological behavior of malignant melanoma cells correlated with their survival in vivo. Cancer Res. 35: 218-244. Gene Therapy Clinical Trials Update - February 1 (2001). The Journal of Gene Medicine, web site at http://www.wiley.co.uk/wileychi/genmed/clinical/charts3.html. Graham, F.L. and Prevec, L. (1991). Manipulation of adenovirus vectors. In: Methods in Molecular Biology (Murray, E.J., ed.). Humana Press, Clifton, NJ, USA, 109-128. Hamburger, V. (1960). A Manual of Experimental Embryology. University of Chicago Press, Chicago, IL, USA. Karnofsky, D.A., Ridgway, L.P. and Patterson, P.A. (1952). Tumor transplantation to the chick embryo. Ann. N. Y. Acad. Sci. 55: 313-329. Knighton, D., Ausprunk, D., Tapper, D. and Folkman, J. (1977). Avascular and vascular phase of tumor growth in the chick embryo. Br. J. Cancer 35: 347-356. Leighton, J. (1964). Invasion and metastasis of heterologous tumors in the chick embryo. Prog. Exp. Tumor Res. 4: 98-125. Norpoth, K. and Rauen, H.M. (1968). Tumorwachstum auf der Chorioallantoismembran im bebrüteten Hühnerei. Z. Krebsforsch. 71: 140-152. Rotman, B. (1961). Measurement of activity of single molecules of b-D-galactosidase. Proc. Natl. Acad. Sci. USA 47: 1981-1991. Stan, A.C., Radu, D.L., Casares, S., Bona, C.A. and Brumeanu, T.D. (1999). Antineoplastic efficacy of doxorubicin enzymatically assembled on galactose residues of a monoclonal antibody specific for the carcinoembryonic antigen. Cancer Res. 59: 115-121. Stratford-Perricaudet, L.D., Makeh, I., Perricaudet, M. and Briand, P. (1992). Widespread long-term gene transfer to mouse skeletal muscles and heart. J. Clin. Invest. 90: 626-630. Zwilling, E. (1959). A modified chorioallantoic grafting procedure. Transplant Bull. 6: 115-116. |