ABSTRACT. Blood plasma of 253 specimens from eight population samples of the sciaenid fish, pescada (Plagioscion squamosissimus), caught from four sites in the Central Amazon, was tested for molecular variants of transferrin. Starch gel electrophoresis was used to distinguish six species of transferrin molecules; 12 of the 21 theoretically possible genotypes were found. There were highly significant departures from genetic equilibrium in seven of the eight population samples (chi-square (c2) test for Hardy-Weinberg expectations) due to an excess of homozygotes and a corresponding deficiency of heterozygotes. A dendrogram based on UPGMA cluster analysis of genetic distances at the transferrin gene locus, estimated among the population samples and statistical analyses of the distribution of Tf allele frequencies, indicated three genetically discreet sub-populations of P. squamosissimus. The three sub-populations, “Careiro/Iranduba”, “Coari” and “Tefe”, were found to have high frequencies of alleles Tf 2, Tf 4 and Tf 3, respectively. This genetic instability may be attributed to genetically discreet “allopatric stocklets”, which diverged during past isolation. Key words: Transferrin polymorphism, Amazon fish, Pescada, Plagioscion squamosissimus
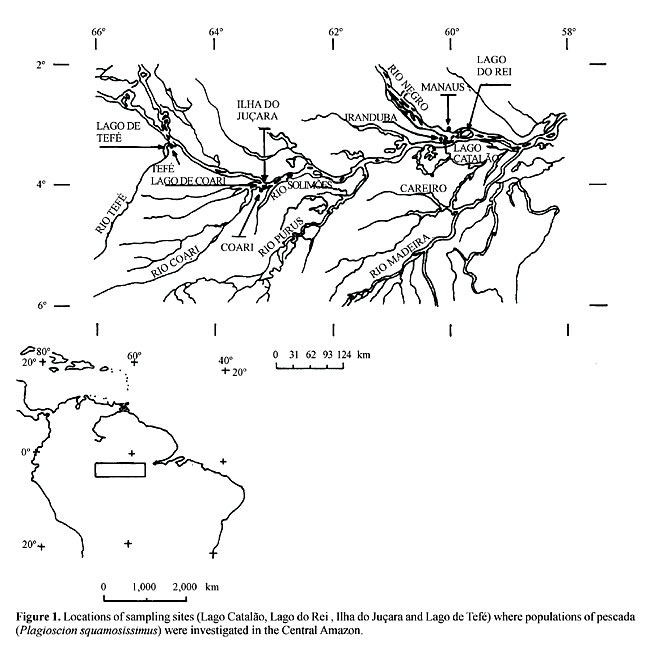
INTRODUCTION The pescada, Plagioscion squamosissimus (Sciaenidae), is a freshwater teleost of commercial importance widely distributed throughout the Amazon region (Soares, 1978). This species is sympatric with another commercial and widely distributed species of pescada (Plagioscion montei) (Soares and Casatti, 2000). These two species are habitually pooled in fishery statistics that are used for commercial stock assessment, although they can be identified by taxonomists through morphometric and meristic characteristics. The migratory patterns of P. squamosissimus in the Amazon region are complex and ill defined, with sporadic and unpredictable movements, as with the characin fishes (Goulding, 1980). Although a few studies have been made on feeding habits, reproductive behaviour, morphology and fishery biology in Lago do Rei, Careiro (Rio Solimões) (Annibal, 1983) and feeding habits in Lago do Janauacá (Rio Solimões) and Lago Aruá (Rio Negro) (Worthmann and Oliveira, 1987), these areas represent only a very minute portion of the Amazon basin. Otolith dimension and fish length data for population samples of P. squamosissimus caught from Lago do Janauacá (Rio Solimões), Lago do Jari (Rio Purus), Arquipélago das Anavilhanas (Rio Negro) and Rio Branco (Worthmann, 1979), as well as cytogenetic studies based on nucleolar organizer region (NOR) heteromorphism, in samples caught from Rio Tefé, Lago Catalão and Rio Pitinga (Feldberg et al., 1999), are evidence that there are distinct populations of this species in the Central Amazon region. Population genetics analyses can reveal the current genetic structure of populations within taxonomic species. Informative gene markers are polymorphic, and effectively independent of selection. The amino acid sites of useful protein markers mutate and allow genetic drift to be measured among populations. An iron binding protein called transferrin is encoded by a single gene locus, Tf, in vertebrate species, most of which have co-dominant Tf alleles. The variants are attributed to single amino acid differences. The transferrin gene locus (Smithies and Hiller, 1959) shows rapidly evolving polymorphic differences, which characterize genetic isolates among numerous vertebrate species (Sarich, 1977; Teixeira and Jamieson, 1985). This locus has proven to be a useful genetic marker for vertebrate populations and/or parentage tests in humans and domestic livestock. Jamieson (1990) compared 157 transferrin alleles in 87 species of teleosts. In the North Atlantic Ocean, the distributions of Tf alleles distinguish regional populations of cod, Gadus morhua (Møller, 1966; Jamieson, 1967; Jamieson and Turner, 1978), and haddock, Melanogrammus aeglefinus (Jamieson and Birley, 1989). Studies of the distributions of transferrin genes in aquatic species in Amazonia have yielded information about the population structure of tambaqui, Colossoma macropomum, in both a wild population (Teixeira and Jamieson, 1985) and in hatchery stocks (Calcagnotto and Toledo-Filho, 2000), in two species of jaraqui, Semaprochiloidus taeniurus and S. insignis (Teixeira et al., 1990), and in a turtle, Podocnemis expansa (Teixeira et al., 1996). We used transferrin gene data to analyze the population structure of a sciaenid fish, pescada (P. squamosissimus), an important food resource in the central Amazon. MATERIAL AND METHODS Two hundred and fifty-three specimens of pescada (P. squamosissimus) were caught from four sampling sites in the Central Amazon: Lago Catalão, Iranduba (confluence of the Rio Negro with Rio Solimões); Lago do Rei, Careiro (Rio Solimões); Ilha do Juçara, Coari (Rio Solimões) and Lago de Tefé, Tefé (Rio Tefé) (Figure 1). Blood was drawn from the dorsal veins of live fish into 5-ml B-D vacutainer tubes, containing 0.5 ml of 3.8% sodium citrate. Blood plasma specimens were separated by centrifugation at 3,500 rpm for 20 min, stored at -25°C, and later thawed for electrophoresis. The plasma was treated with 2% rivanol solution at a ratio of 1:2 to isolate plasma transferrin molecules prior to electrophoresis (Jamieson and Turner, 1978).
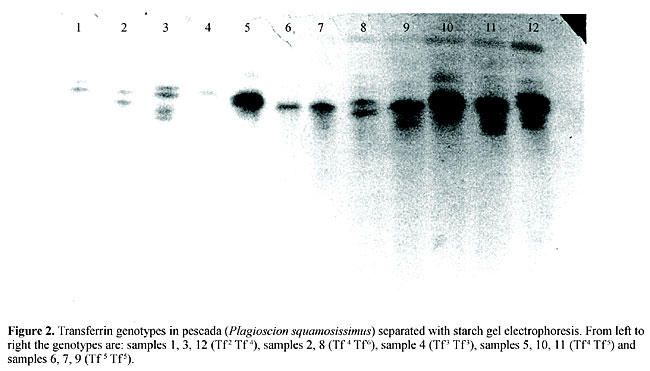
The electrophoresis procedures, including the preparation of gel-electrode buffer systems (Tris-citrate, lithium hydroxide-boric acid), preparation of starch gel medium, sample application and gel staining, followed Jamieson and Turner (1978). The gel slices were immersed several times in a vessel containing a solution of 5:5:1 parts of water, methanol andacetic acid, respectively, for approximately 15 h to elute excess stain. Six putative co-dominant Tf alleles were numerically classified according to their decreasing electrophoretic mobilities (Tf 1, Tf 2, Tf 3, Tf 4, Tf 5 and Tf 6). Several pescada (P. squamosissimus) plasma controls of known genotypes were selected and interspersed along the gels to compare, match and read the different genotypes. A computer program: “Tools for Population Genetic Analyses” (TFPGA), recently developed by Miller (1997), was used to analyze Tf locus polymorphism with descriptive statistics, Hardy-Weinberg equilibrium tests, Rogers (1972) genetic distance modified by Wright (1978), UPGMA cluster analyses using genetic distance measures and exact tests for population differentiation (Raymond and Rousset, 1995). Contingency tests were also applied to examine the frequency distribution of the most common transferrin alleles scored in the putative sub-populations of pescada. RESULTS Transferrin genotypes in pescada Twelve genotypes (Tf 1 Tf 1, Tf 1 Tf 2, Tf 2 Tf 2, Tf 2 Tf 4, Tf 2 Tf 6, Tf 3 Tf 3, Tf 3 Tf 4, Tf 3 Tf 6, Tf 4 Tf 4, Tf 4 Tf 5, Tf 4 Tf 6 and Tf 5 Tf 5) of 21 theoretically possible ones, were detected, encoded by six co-dominant alleles (Tf 1, Tf 2, Tf 3, Tf 4, Tf 5 and Tf 6) (Table 1). Individual homozygote fish produced a single transferrin band, whereas individual heterozygote fish showed two transferrin bands (Figure 2). This monomeric structure of transferrin molecules, with single-locus co-dominant inheritance, is homologous with the variation observed at the transferrin locus in all of the many vertebrate species tested.
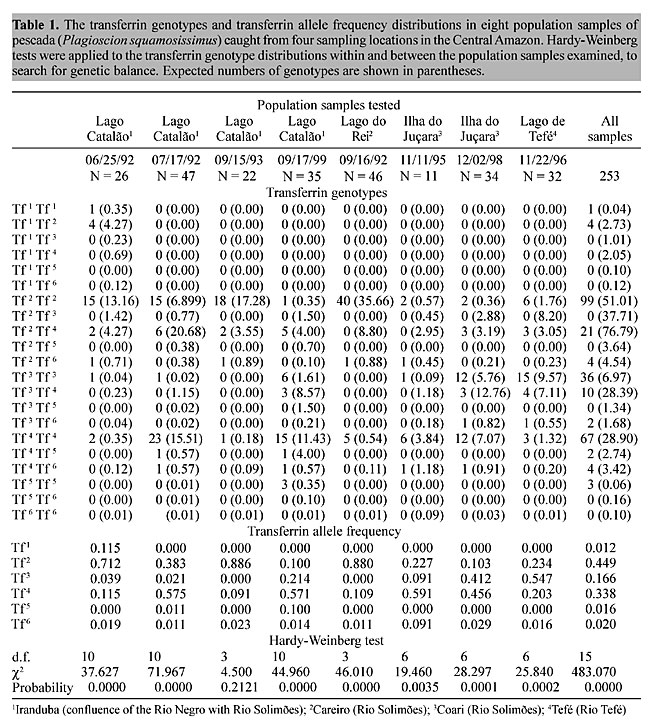
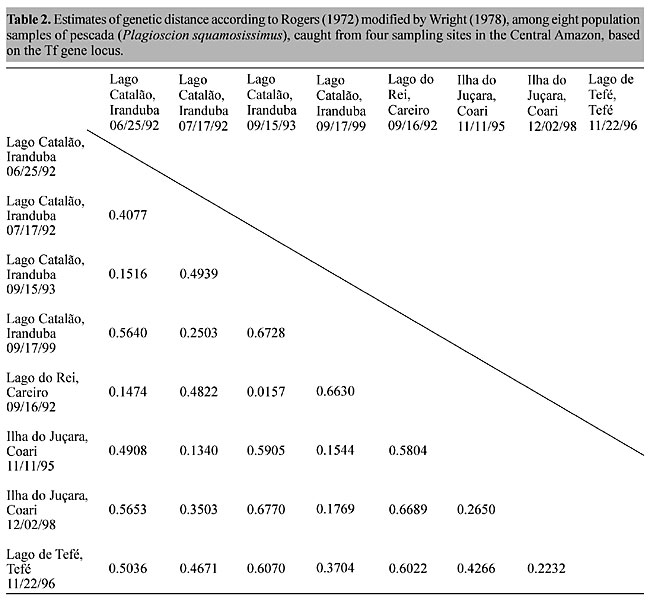
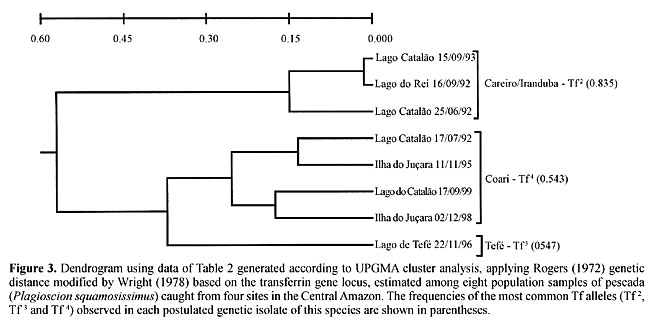
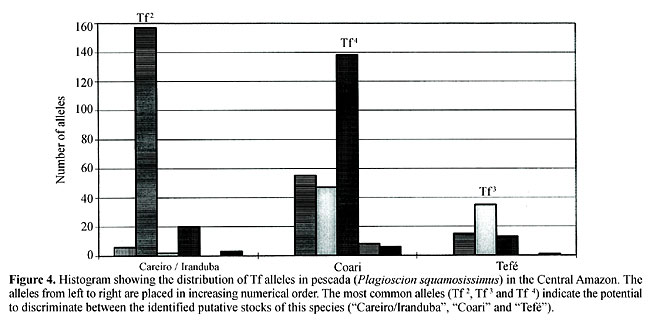
Regional variations in transferrin allele and genotype distribution To test for genetic balance in pescada (P. squamosissimus) population samples from each area, c2 tests for Hardy-Weinberg expectations were applied (Table 1). The c2 values revealed highly significant departures from genetic equilibrium in seven of the eight population samples examined. There was an excess of homozygotes and corresponding deficiencies of heterozygotes. The only example of genetic equilibrium was found in the Lago Catalão, Iranduba 09/15/93 population sample (c2(3) = 4.50, P = 0.21). The strong degree of genetic imbalance found was mainly due to the excess of homozygotes Tf 3 Tf 3 and Tf 4 Tf 4 in the Lago Catalão, Iranduba 06/25/92 population sample, excess of homozygotes Tf 2 Tf 2 and Tf 3 Tf 3 in the Lago Catalão, Iranduba 07/17/92 and in the Ilha do Juçara, Coari 11/11/95 population samples, excess homozygotes Tf 3 Tf 3 and Tf 5 Tf 5 in the Lago Catalão, Iranduba 09/17/99 population sample, excess homozygotes Tf 2 Tf 2, Tf 3 Tf 3 and Tf 4 Tf 4 observed in the Ilha do Juçara, Coari 12/02/98 and in the Lago de Tefé population samples, and excess of the homozygote Tf 4 Tf 4 and deficiency of the heterozygote Tf 2 Tf 4 observed in the Lago do Rei, Careiro 09/16/92 population sample. There was also considerable disequilibrium in the grand total sample. Exact tests for population differentiation (Raymond and Rousset, 1995) in a simultaneous analysis of all population samples also indicated highly significant differences (P < 0.001). A matrix was produced by applying Rogers (1972) genetic distance modified by Wright (1978) to our transferrin data (Table 2). A dendrogram generated based on UPGMA cluster analysis using the data of Table 2 (Figure 3) shows three genetically distinct sub-populations of P. squamosissimus in the sampled areas: “Careiro/Iranduba” sub-population comprising the population samples: Lago Catalão, Iranduba 09/15/93, Lago do Rei, Careiro 09/16/92 and Lago Catalão, Iranduba 06/25/92; “Coari” sub-population, comprising the population samples: Lago Catalão, Iranduba 07/17/92, Ilha do Juçara, Coari 11/11/95, Lago Catalão, Iranduba 09/17/99 and Ilha do Juçara, Coari 12/02/98; and “Tefé” sub-population, comprising the population sample: Lago de Tefé 11/22/96. In the “Careiro/Iranduba” sub-population the most common transferrin allele was Tf 2, with a frequency of 0.835 (ranging from 0.712 to 0.886). The most common transferrin allele in the “Coari” sub-population was Tf 4, with a frequency of 0.543 (ranging from 0.456 to 0.591). In the dendrogram shown in Figure 3, two population samples caught from Lago Catalão: Iranduba 07/17/92 and Iranduba 09/17/99 appear clustered together with the population samples of the postulated “Coari” sub-population, while it was expected that they would appear clustered together with the population samples of the postulated “Careiro/Iranduba” sub-population. The “Tefé” sub-population was characterized by its most common allele (Tf 3), with a frequency of 0.547. The most common Tf alleles (Tf 2, Tf 3 and Tf 4) are potential genetic markers to discriminate sub-populations of pescada (P. squamosissimus) in the Central Amazon (Figure 4).
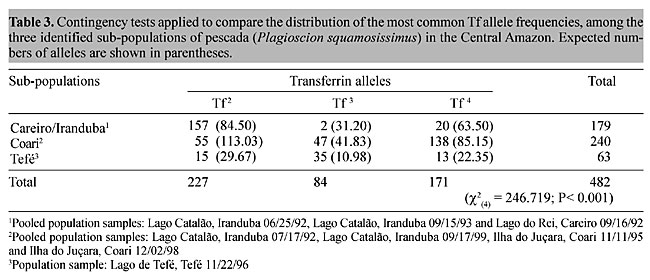
There were highly significant differences between the frequency distributions of the most common transferrin alleles (Tf 2, Tf 3 and Tf 4) among the discreet sub-populations of pescada (“Careiro/Iranduba”, “Coari” and “Tefé”) (Table 3). The frequency distributions of the
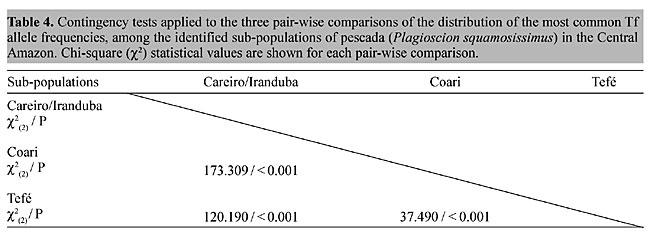
most common alleles among the population samples belonging to the postulated “Careiro/Iranduba” sub-population (Lago Catalão, Iranduba 06/25/92, Lago Catalão, Iranduba 09/15/93 and Lago do Rei, Careiro 09/16/92), were relatively homogeneous (c2(4) = 6.538; 0.20 > P > 0.10), in contrast with the high degree of heterogeneity found for the postulated “Coari” sub-population (Lago Catalão, Iranduba 07/17/92, Lago Catalão, Iranduba 09/17/99, Ilha do Juçara, Coari 11/11/95 and Ilha do Juçara, Coari 12/02/98) (c2(6) = 54.069; P < 0.001). Contingency tests on the distribution of the most common transferrin allele, applied to the three comparisons which represent all possible pair-wise combinations among the identified pescada sub-populations (“Careiro/Iranduba” x “Coari”; “Careiro/Iranduba” x Tefé” and “Coari” x “Tefé”), revealed highly significant differences (Table 4).
DISCUSSION An excess of homozygotes in population samples is usually due to one or more among four factors: 1) mixing of genetically distinct populations, known as the “Wahlund effect” (Wahlund, 1928), 2) null allele leading to a false observation of excess of homozygotes (however, null alleles at this Tf gene locus are extremely rare), 3) inbreeding, and 4) gene locus under selection. Excess of homozygotes for esterase polymorphisms in marine teleosts has been reported, but without adequate explanations (see Smith et al., 1981). An excess of homozygotes observed for a serum esterase locus in the Atlantic mackerel (Scomber scombrus) might be a consequence of shifting selection on different batches of larvae produced in different regions of the spawning area and in different seasons (Smith et al., 1981). Whereas, excess of homozygotes in hemoglobin polymorphism in Atlantic cod, Gadus morhua L., leading to a considerable regional and seasonal variation in allele frequencies, was attributed to the “Wahlund effect” (Jamieson and Birley, 1989). Further detailed sampling may explain the observed genetic imbalance in pescada (P. squamosissimus) due to an excess of Tf homozygotes, and may indicate a genetic strategy peculiar to this species. Despite the lack of a precise migratory pattern of P. squamosissimus in the Amazon basin (Goulding, 1980), the presence of two population samples caught from Lago Catalão: Iranduba 17/07/92 and Iranduba 17/07/99, which clustered together with the population samples of the postulated “Coari” sub-population (Figure 3), might be attributed to downstream drift of eggs or pelagic larvae from Coari, Rio Solimões to the vicinity of Lago Catalão (confluence of the Rio Negro with the Rio Solimões). If this is a correct assumption, at least two questions can now be made: 1) How long do the fishes remain at Lago Catalão, from the time that the eggs or pelagic larvae got there? 2) Do the adult individuals return upstream to their natal source? Currently, the Lago Catalão is widely considered a stopping place and passage corridor in the migratory route of fish species in the Central Amazon. This could certainly be tested in pescada and in other migratory species, by using genetic tags to make spatial and temporal comparisons of gene and genotype frequencies in the Central Amazon. Statistical analyses on Tf allele frequency distributions indicated the existence of three genetically discreet sub-populations of pescada (P. squamosissimus) in the surveyed areas. Our findings are partly supported by data on relationships between otolith dimensions and fish length (Worthmann, 1979) and nucleolar organizer region (NOR) heteromorphism (Feldberg et al., 1999), which indicate a possible occurrence of distinct populations of this species in the Central Amazon. Genetic polymorphism is widespread, and provides opportunities to test the genetic stability of species. A model biological species is an interbreeding unit, in which random mating produces genotypes in predictable proportions, i.e., according to Hardy-Weinberg expectations. As most of the tested material failed to comply with this null hypothesis, we may conclude that the fish we sampled did not mate at random. A conventional interpretation assumes that genetic polymorphism is maintained by natural selection favouring heterozygosity. There is reason to think that this applies on an evolutionary time scale. It is intellectually comfortable to speculate on the possible selective advantage of particular heterozygotes, but it is difficult to find practical examples in current population surveys. The frequencies of pescada Tf genotypes found indicate quite the opposite of heterozygote advantage. This could be due to random events, as follows. Many species are composed of numerous sub-populations, which can be, more or less, in genetic isolation. The contemporary populations are the chance products of previous populations. Occasional reductions in the effective numbers of founder parents can have a bottleneck effect on the chance distribution of the alleles in their descendants. The pescada samples could be the immediate products of historic bottlenecks. To produce such a set of data in a model, it is necessary to pool population samples with very diverse distributions of particular alleles. The present form of the Amazon basin is explained by a historic reversal of the main drainage outflow, originally to the Pacific, and presently to the Atlantic. Consequent to this change, numerous lacustrine habitats were formed during the Miocene, influencing the diversification of fishes (Goulding 1980; Lundberg et al., 1998). The genetic instability that we found in pescada, P. squamosissimus, may be attributed to genetically discreet “allopatric stocklets” that diverged during past isolation. To satisfy this “bottleneck” interpretation it would be necessary to discover examples of populations showing balanced genotypes, at least one with a high frequency of allele Tf 3 and another with a high frequency of the allele Tf 4. A balanced population showing high Tf 2 was found on 09/15/93 at Lago Catalão. ACKNOWLEDGMENTS The authors thank Dr. Eliana Feldberg and Dr. Jorge I.R. Porto for discussion and encouragement, Mr. Sigueru A. Esashika and Mr. Agenor N. da Silva for catching fish samples, Mr. Raimundo S. da Silva for helping in the collection of blood samples and Mr. Felipe F. Moraes for drawings and for photographing gels. Research partially supported by INPA (National Institute for Research in the Amazon) through the Institutional Project Programme (PPI 3-3270 and PPI 1-3090). REFERENCES Annibal, S.R.P. (1983). Avaliação bio-ecológica e pesqueira das “pescadas” (Plagioscion squamosissimus HECKEL, 1840 e Plagioscion montei SOARES, 1978) no “Sistema Lago do Rei”- Ilha do Careiro - AM - Brasil. Master thesis, PPG-BTRN, INPA, Manaus, AM, Brazil. Calcagnotto, D. and Toledo-Filho, S.A. (2000). Loss of genetic variability at the transferrin locus in five hatchery stocks of tambaqui (Colossoma macropomum). Genet. Mol. Biol. 23: 127-130. Feldberg, E., Porto, J.I.R., dos Santos, E.B.P. and Valentin, F.C.S. (1999). Cytogenetic studies of two freshwater sciaenids of the genus Plagioscion (Perciformes, Sciaenidae) from the Central Amazon. Genet. Mol. Biol. 22: 351-356. Goulding, M. (1980). The Fishes and the Forest, Explorations in Amazonian Natural History. University of California Press, Berkeley, CA, USA. Jamieson, A. (1967). New genotypes in cod at Greenland. Nature 215: 661-662. Jamieson, A. (1990). A survey of transferrin in 87 teleostean species. Anim. Genet. 21: 295-301. Jamieson, A. and Birley, A.J. (1989). The distribution of transferrin alleles in haddock stocks. J. Cons. Int. Explor. Mer 45: 248-262. Jamieson, A. and Turner, R.J. (1978). The extended series of Tf alleles in Atlantic cod, Gadus morhua. In: Marine Organisms (Battaglia, B. and Beardmore, J.A., eds.). Plenum Publishing Corporation, New York, NY, USA, pp. 699-729. Lundberg, J.G., Marshall, L.G., Gerrero, J., Horton, B., Malabarba, M.C.S.L. and Wesselingh, F. (1998). The stage for neotropical fish diversification: A history of tropical South American rivers. In: Phylogeny and Classification of Neotropical Fishes (Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M.S. and Lucena, C.A.S., eds.). EDIPUCRS, Porto Alegre, RS, Brazil, pp. 13-48. Miller, M.P. (1997). Tools for Population Genetic Analyses (TFPGA) 1.3: A Windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author. Møller, D. (1966). Genetic differences between cod groups in the Lofoten area. Nature 212: 824 (Abstract). Raymond, M.L. and Rousset, F. (1995). An exact test for population differentiation. Evolution 49: 1280-1283. Rogers, J.S. (1972). Measures of genetics similarity and genetic distance. In: Studies in Genetics VII. University of Texas, Austin, TX, USA. Publication No. 7213, pp. 145-154. Sarich, V.M. (1977). Rates, sample sizes, and the neutrality hypothesis for electrophoresis in evolutionary studies. Nature 265: 24-28. Smith, P.J., Francis, R.I.C.C. and Jamieson, A. (1981). An excess of homozygotes at a serum esterase locus in the Atlantic mackerel Scomber scombrus. Anim. Blood Groups Biochem. Genet. 12: 171-180. Smithies, O. and Hiller, O. (1959). The genetic control of transferrins in humans. Biochem. J. 72: 121-126. Soares, L.H. (1978). Revisão taxonômica dos scianídeos de água doce da região amazônica brasileira (Osteichthyes, Perciformes, Sciaenidae). Masters thesis, PPG-BTRN, INPA, Manaus, AM, Brazil. Soares, L. and Casatti, L. (2000). Descrição de duas novas espécies de Sciaenidae (Perciformes) de água doce da bacia amazônica. Acta Amazonica 30: 499-514. Teixeira, A.S. and Jamieson, A. (1985). Genetic variation in plasma transferrins of tambaqui, Colossoma macropomun (CUVIER 1818). Amazoniana 9: 159-168. Teixeira, A.S., Raposo, J.C.P. and Jamieson, A. (1990). Transferrin variation in jaraquis, Semaprochilodus taeniurus and S. insignis, in the Amazon region. Anim. Genet. 21: 419-422. Teixeira, A.S., Jamieson, A., Raposo, J.C.P. and Vieira, A.A. (1996). Transferrin polymorphism in Amazon turtle (Podocnemis expansa) stocks. Braz. J. Genet. 19: 559-564. Wahlund, S. (1928). Zusammensetzung von Populationen und Korrelationsers-chinungen von Standpunkt der Vererbungslehre aus betrachtet. Hereditas 11: 65-108. Worthmann, H. (1979). A relação entre o desenvolvimento do otólito e o crescimento do peixe como auxílio na distinção de populações de pescada (Plagioscion squamosissimus). Acta Amazonica 9: 573-586 Worthmann, H. and Oliveira, J.L. (1987). Comparative nutritional analysis of two sciaenidian species, the pescadas, Plagioscion squamosissimus HECKEL and Plagioscion monti SOARES, from different water systems of the Central Amazon. Animal Research and Development. A Biannual Collection of Recent German Contributions concerning Development through Animal Research, 25: 7-34. Wright, S. (1978). Evolution and the genetics of populations. Vol. 4. Variability within and among natural populations. University of Chicago Press, Chicago, IL, USA. |