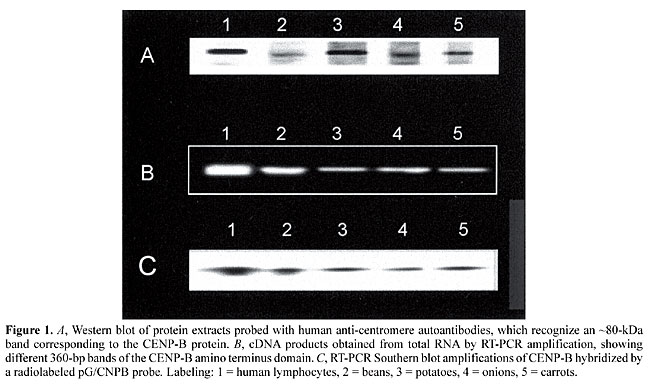
ABSTRACT. To explore the CENP-B centromere protein in beans, carrots, onions and potatoes, total RNA was isolated and reverse transcribed by PCR, and the cDNA encoding the CENP-B amino terminus domain amplified using CENP-B oligonucleotides. Blots containing PCR products were hybridized with a nick-translated pG/CNPB probe containing a complete human CENP-B gene. In all the plant species, anti-CENP-B antibodies recognized an 80-kDa protein. A 360-bp sequence encoding for the amino terminus region of the CENP-B protein was amplified by PCR in all the species and the nick translated pG/CNPB probe hybridized with the PCR products. Apparently the CENP-B centromere protein or an equivalent protein is widely distributed in the vegetal kingdom. Key words: Centromere formation, CENP-B gene, CENP-B protein, DNA isolation, Vegetal species INTRODUCTION The centromere is a specialized region of eukaryotic chromosomes, composed of centromeric (kinetochore) DNA and proteins, both involved in forming the centromere/kinetochore complex, which drives the segregation of condensed chromosomes during mitosis (Earnshaw and Mackay, 1994). According to Masumoto et al. (1989), heterochromatin contains DNA repeats or a-satellite arrays formed by 171-bp monomers, which possess a 17-bp protein binding motif (CENP-B box) that binds to a monomer of CENP-B protein. In mammalian centromeres CENP-B plays a critical role in the organization of chromatin structure, but in the absence of CENP-B some chromosomes use other redundant proteins to accomplish mitosis (Pluta et al., 1992; Kipling et al., 1995). CENP-B is the target of human autoimmune sera from patients with a form of CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, and telangiectasia) scleroderma. Moroi et al. (1980) have shown that centromeric proteins, including CENP-B, can be characterized using sera from CREST patients. Human sera from scleroderma patients have been used to localize plant centromeres by indirect immunofluorescence (Mole-Bajer et al., 1990). The amino terminus domain of a CENP-B encoding gene in Phaseolus vulgaris has been amplified by the reverse transcriptase-PCR (RT-PRC) method and CENP-B box has been in situ hybridized in dissociated plant cells (Barbosa-Cisneros et al., 1997). We investigated the CENP-B gene in various plant species. MATERIAL AND METHODS Plant growth conditions and protein and DNA isolation Bean, potato, carrot and onion seeds were planted in Murashige-Skoog gel media. RNA and protein were extracted 2 to 6 weeks after planting by collecting 2 g of roots, which were then ground in a mortar with liquid nitrogen and dissolved in 10 ml of 10 mM Tris, pH 7.4, containing 10 mM NaCl, 25 mM EDTA and 1 ml of 10% SDS. The mixture was incubated with 1 mg/ml proteinase K for 2 h at 37°C and 1 ml of 5 mM NaCl was added. The proteins obtained were separated by electrophoresis, electrotransferred and probed with CREST autoantibodies using the Western blot technique. Nick translation The CENP-B gene was excised using the Sma restriction enzyme from the pG/CNPB vector and nick translated with 10 ml of unlabeled triphosphate dGTP, dATP, dTTP mix and 4 ml of 32P-dCTP (Amersham) in 2.5 ml of nick translation buffer containing 0.5 ml of DNA polymerase 1. The reaction mixture was incubated at 15°C for 2 h and the labeled probe precipitated with 3 M sodium acetate, SS-phenol and chloroform and adjusted to a specific activity of 1 x107 cpm (Davies et al., 1986). Oligonucleotide probes for the CENP-B gene Oligomer synthesis was performed in an Applied Biosystems DNA synthesizer. Probes were de-protected and cleaved and then purified from cartridges using ammonium hydroxide, HPLC grade acetonitrile, trifluoroacetic acid and acetonitrile buffers. Oligomers were synthesized using the human CENP-B sequence (Earnshaw et al., 1987) 5´-CGACAGCTGACGTTCCGGGA-3´ (upstream) and 5´-CGCACCACACAGGACGTCGCCGCACC-3´ (downstream). Reverse-transcriptase and PCR Total RNA was extracted from vegetal tissues by the acid guanidium thiocyanate/phenol/chloroform method (TRlzol, Gifco, BRL) and 250 ng of this RNA incubated with 200 mM of DNTP, 0.75 mM of downstream primer and 5 U/20 ml rTth/DNA polymerase (Gene Amp ™PCR system 9600). The reverse transcriptase reaction was performed at 70°C for 10 min. The PCR reaction was carried out in a thermal cycler (COY TempCycler) using 30 cycles at 94°C for 2 min, 48°C for 2 min and 72°C for 1.4 min, after the addition of a 0.15 mM upstream primer. PCR products were separated by electrophoresis in 0.8% ethidium bromide agarose (Wang and Mark, 1990). Southern blot analysis Gels were denatured for 60 min in 10 M NaNOH and 5 M NaCl, and neutralized in 10 M ammonium acetate and 10 M NaOH. DNA was transferred onto nylon membranes in 10X SSC and kept overnight, after which the blots were heated at 80°C for 2 h and pre-hybridized for 15 min at 65°C in rapid-hyb buffer (Amersham). The probe was denatured at 95°C for 2 min, and adjusted in hybridization buffer to 1 x 107 cpm, placed into a bag, which was heat-sealed and incubated overnight in a water bath at 42°C. The blots were then washed in 2X SSC and 0.1% (w/v) SDS at room temperature for 20 min and then twice in 1X SSC and 0.1% (w/v) SDS at 42°C for 15 min. The blots were dried, wrapped and autoradiographed for 3 h at -70°C using Kodak X-OMAT AR film and screen. RESULTS CENP-B protein was detected by anti-CENP/B antibody Western blots in all the plant species tested, the plant protein being molecularly similar to human CENP/B protein with a molecular mass of ~80 kDa (Figure 1). Using total RNA and RT-PCR amplification it was possible to amplify a 360-bp sequence of the CENP-B amino terminus domain, which was separated by electrophoresis, blotted and probed with pG/CNPB using Southern blot analysis. The nick translated pG/CNPB probe hybridized the RT-PCR plant products. Human CENP-B cDNA from peripheral lymphocytes used as controls was also hybridized in situ by pG/CNPB, the cDNA human bands behaving similarly to those from plant DNA (Figure 1).
DISCUSSION Kinetochore proteins are involved in the general maintenance of centromeres, with CENP-B protein being mainly engaged in centromere formation. It is widely distributed in nature. In yeasts the autonomously replicating sequence-binding protein (Abp1p) is identical to CENP-B, and it is known that Abp1p participates in chromosome segregation, suggesting that yeast fission centromeres are structurally and functionally related to centromeres from higher eukaryotes (Halverson et al., 1977). In plants, the CENP-B box sequences are present in cereals (Triticeae, maize and rice), while CCS1 sequences also exhibit a high degree of homology with CENP-B box (Aragon-Alcaide et al., 1996). Earlier studies have also shown that the CENP-B protein is present in Phaseolus vulgaris (Barbosa-Cisneros et al., 1997), and since CENP-B box is present in plants, we inferred the presence of the CENP-B protein as a CENP-B box counterpart. We detected proteins similar to the Phaseolus CENP-B protein in beans, carrots, onions and potatoes using the strategy proposed by Sugimoto and Himeno (1991). Apparently the CENP-B protein is a conserved molecule in plant genomes. ACKNOWLEDGMENTS The pG/CNPB vector was kindly donated to us by Ann Pluta from Johns Hopkins University, USA. Research supported by CONACYT grant 1877 PM and scholarship 92261. REFERENCES Aragon-Alcaide, L., Miller, T., Schwarzacher, T., Reader, S. and Moore, G. (1996). A cereal centromeric sequence. Chromosoma 105: 261-268. Barbosa-Cisneros, O., Fraire-Velazquez, S., Moreno, J. and Herrera-Esparza, R. (1997). CENP-B autoantigen is a conserved protein from humans to higher plants: Identification of the aminoterminal domain in Phaseolus vulgaris. Rev. Rhum. Engl. Ed. 64: 368-374. Davies, L.G., Dibner, M.D. and Battey, J.F. (1986). Preparation of DNA from eukaryotic cells: general method. In: Basic Methods in Molecular Biology. Elsevier, Amsterdam, Netherlands, pp. 44-61. Earnshaw, W.C. and Mackay, A. (1994). Role of nonhistone proteins in chromosomal events of mitosis. FASEB J. 8: 947-956. Earnshaw, W.C., Sullivan, K.F., Machin, P.H., Cooke, C.A., Kaiser, D.A., Pollard, T.D., Rothfield, N.F. and Cleveland, D.C. (1987). Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J. Cell Biol. 104: 817-829. Halverson, D., Baum, M., Strykrr, J., Carbon, J. and Clarke, L. (1977). A centromere DNA-binding protein from fission yeast affects chromosome segregation and has homology to human CENP-B. J. Cell Biol. 136: 487-500. Kipling, D., Mitchell, A.R., Masumoto, H., Wilson, H.E., Nicol, L. and Cooke, H.J. (1995). CENP-B binds a novel centromeric sequence in the Asian mouse Mus caroli. Mol. Cell Biol. 15: 4009-4020. Masumoto, H., Masukata, H., Muro, Y. and Okazaki, T. (1989). A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J. Cell Biol. 109: 1963-1973. Mole-Bajer, J., Bajar, A.S., Zinkowsky, R.P. and Brinkley, B.R. (1990). Autoantibodies from patients with sclerodemma CREST recognized kinetochores of the higher plant Haemantus. Proc. Natl. Acad. Sci. USA 87: 3599-3603. Moroi, Y., Pables, C., Fritzler, M.J., Steirgerwald, J. and Tan, E.M. (1980). Autoantibody to centromere (kinetochore) in sclerodemma sera. Proc. Natl. Acad. Sci. USA 77: 1627-1631. Pluta, A.F., Saitoh, N., Goldberg, I. and Earnshaw, W. (1992). Identification of a subdomain of CENP-B that is necessary and sufficient for localization to the human centromere. J. Cell Biol. 116: 1081-1093. Sugimoto, K. and Himeno, M. (1991). A rapid isolation of the unknown 5'-flanking sequence of human CENP-B cDNA with polymerase chain reaction. Agric. Biol. Chem. 55: 2687-2692. Wang, A.M. and Mark, D.F. (1990). Quantitative PCR. In: PCR Protocols. A Guide to Methods and Applications. (Innis, M.A., Gelfand, D.H., Sninsky, J.J. and White, T.J., eds.). 1st edn. Academic Press, New York, NY, USA, pp. 70-75. |