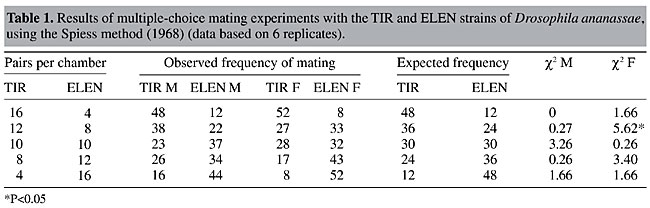
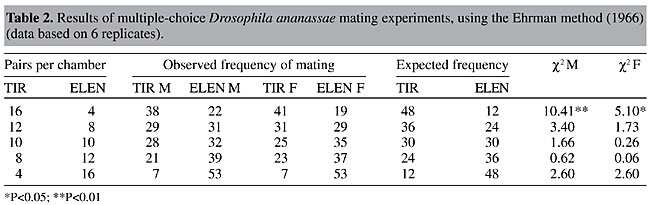
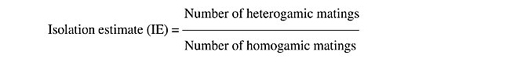ABSTRACT. Minority male mating advantage was tested in wild type strains of Drosophila ananassae through multiple-choice experiments. Mating success of two types of flies present in five different ratios was scored by direct observation in an Elens-Wattiaux mating chamber. We found no evidence for minority male mating advantage in wild type strains of D. ananassae. The relative mating success of two types of females was also compared in the multiple-choice experiments at different ratios; there was no evidence for a rare female effect. Further, there was similarity in the results of experiments employing different methods. The total number of homogamic and heterogamic matings was obtained by combining the data (all five ratios) from each experiment. Homogamic matings were significantly more frequent than heterogamic ones, which demonstrates preferential mating between males and females of the same strain; this was also supported by a lower isolation estimate. There was also a significant difference in the degree of mating preference between the two strains. Key words: Drosophila ananassae, Homogamic matings, Heterogamic matings, Mating preference, Multiple-choice experiments INTRODUCTION Rare male mating advantage, one of the best studied examples of frequency-dependent fitness, has been extensively studied in Drosophila and also to some extent in other invertebrates, and in a few vertebrates, since it was first demonstrated in population genetics studies (Petit, 1951). The term “rare male mating advantage” implies that when two variants (phenotypic or genotypic) of the same species are present together, the relative mating success of males of each of the variants is inversely related to that variant’s relative abundance in the population, i.e., the rare male type has a higher rate of mating success than the common type. Rare male advantage is especially important as, in contrast with the classical overdominance model, it can maintain high levels of genetic variability in natural populations with almost total absence of genetic load at equilibrium. It also promotes outbreeding and gene exchange among populations (Averhoff and Richardson, 1974; Lewontin, 1974; Dal Molin, 1979; Grant et al., 1980). Studies on rare male mating advantage in 12 different species of Drosophila and on a variety of other organisms have been summarized in reviews by Knoppien (1985a) and Singh and Sisodia (2000). Though there is strong evidence for the rare male effect, it is not unanimously accepted. Questions have been raised regarding what actually causes it, and even concerning its existence. Several authors (Bryant et al., 1980; Markow, 1980; Knoppien, 1985b) have criticized the methodology of experiments made to test for this phenomenon, as they may result in spurious rare male advantages. As Lewontin (1974) points out, since almost every male is rare for quite a few genes, the advantage of rarity at various loci would cancel, leading to a questionable net effect. Anderson and Brown (1984) stated that the prevalence of a rare male advantage is probably overstated in the literature as experiments showing negative results were not always published, and hence have not come to light. In D. ananassae, a cosmopolitan and domestic species, unique among the species of Drosophila for its possession of a number of genetic peculiarities (Singh, 1985, 1996, 2000; Tobari, 1993), a number of studies have been made on certain aspects of behavior (Markow and Smith, 1979; Singh and Chatterjee, 1985, 1986, 1987, 1988, 1989; Singh and Pandey, 1993a,b; Joshi, 1999; Singh and Singh, 1999, 2000). Rare male mating advantage was detected in this species by comparing cd and se mutants with wild type flies (Singh and Chatterjee, 1989). In the above context, to see the ubiquity of this phenomenon in D. ananassae, rare male experiments were carried out earlier in two wild type strains of D. ananassae, derived from different geographic localities by employing a female-choice method with two different experimental designs as well as different marking procedures (Som and Singh, 2001). There was no evidence for rare male mating advantage. Further, the high degree of consistency in the results with different experimental designs gives evidence that experimental designs have no effect on the results. Bryant et al. (1980) reported that if males are harmed during marking for identification, such that their mating success is subsequently affected, a rare male effect would be enhanced. In their experiments they marked the flies by wing clipping. So, two different marking procedures (clipping distal part of the right wing or putting a small drop of nail polish on the thorax) were used by Som and Singh (2001). But different marking procedures also had no effect on the outcome of these experiments. They were conducted employing a “female-choice” technique in which one type of female was placed together with two different types of males, at different ratios. Mating behavior in Drosophila can be tested by single-pair matings, in which one type of male is placed with one type of female, i.e., a “no choice” experiment, by “male-choice” matings, where one type of male is confined with two types of females, by “female-choice” matings, where one type of female is placed with two types of males or by “multiple-choice” experiments in which both types of males and females are confined together. The rare male effect can be detected by “female-choice” or “multiple-choice” experiments. However, multiple-choice experiments appear to have some advantages over female-choice trials to examine the rare male effect, as they permit the observations of four types of matings, i.e., A female x A male, A female x B male, B female x A male, B female x B male (A and B denote two types of flies). Also this method gives the closest approach to natural population conditions. We tested the rare male effect in wild type strains of D. ananassae with multiple-choice experiments. MATERIAL AND METHODS Two different wild type strains of D. ananassae, kept in mass culture, were used (see Som and Singh, 2001). They were: TIR - established from flies collected from Tirupati (Andhra Pradesh, India) in 1990 and ELEN - initiated with flies collected in Elenthikara (Kerala, India) in 1993. In a preliminary test, mating propensities of these two strains were found to be more or less equal. The stocks were maintained on standard fruit fly medium. Virgin flies from both the strains were separated by sex under light ether anesthesia within a few hours of eclosion to avoid previous exposure of females to male courtship, which may otherwise affect results (Pruzan, 1976). Virgin females and males were stored in separate food vials in batches of 15. This number was fixed to avoid bias due to density effects (Knoppien, 1985b). Flies were aged for seven days. One day before experiment, both sexes were marked under light ether anesthesia by clipping the distal part of the right wing. In half of the replicates, flies of one strain were marked, and in the other half flies of the other strain were marked. The two strains were marked in alternate runs, i.e., by rotating marking from strain to strain in the various replicate mating trials of a cross in order to nullify adverse effects of marking, if any. Though this might not be an ideal way to balance the adverse effects of marking when the two competing male types are present in unequal frequencies (Bryant et al., 1980), it was applied in the same way to both the strains. As different marking techniques do not normally affect mating propensity (Robertson, 1982; Som and Singh, 1998), we used only one marking procedure. After marking, 20 females and 20 males were kept separately in fresh food vials at the ratios to be tested. Females and males of the two strains were taken at five different ratios (4:16, 8:12, 10:10, 12:8 and 16:4). The ratio of males to females varied simultaneously. Care was taken to avoid sampling error (Markow, 1980). Six replicates were carried out for each ratio. Two experimental methods were employed, named here as “Spiess method” (Spiess, 1968) in which after commencement of mating, mated pairs were aspirated out and later identified, and the “Ehrman method” (Ehrman, 1966), where the flies were not removed and the types of males and females in the mating pairs were observed in the mating chamber. Spiess and Ehrman used different experimental conditions, e.g., multiple-choice or female-choice technique, different experimental ratios, different sex ratios, etc. (Spiess et al., 1966; Ehrman, 1966, 1968; Spiess, 1968, Spiess and Kruckeberg, 1980; Spiess and Bowbal, 1987). However, for convenience, two generalized terms, “Spiess method” and “Ehrman method” are used here which differ basically in the way of observation (detecting the type of copulating pair either by taking out or within the chamber itself). In the former method, individual flies cannot mate more than once, while in the Ehrman method remating is possible. Flies were introduced into an Elens-Wattiaux mating chamber (Elens and Wattiaux, 1964) without etherization. First 20 females were placed in the chambers, and then 20 males. The sex ratio was always 1:1, i.e., one female for every male. The first 10 matings were recorded in each run. In Spiess method, copulating pairs were aspirated out into separate empty vials and later identified and their sequences were noted, while in Ehrman method, type of mated flies and their sequences were noted directly from mating chamber by the use of 4X hand lens. The tests were performed from 7:00 to 10:30 am in a temperature-controlled room (about 24oC), under normal laboratory lighting conditions (about 10,000 lux). Six hundred matings were scored for each method (10 matings in each run, with 6 replications of 5 ratios). RESULTS The expected numbers of matings were calculated on the basis of the ratio between the two types of males (or females) introduced into the mating chamber (Tables 1 and 2). In most cases, the c2 values were nonsignificant (P>0.05). In only one case, when ELEN males were rare (4) to 16 TIR males, there was a significant rare male mating advantage (Table 2). The c2 values for females were also calculated and a significant rare female mating advantage (P<0.01) for ELEN females was also found in the same group of tests. In another trial with 8 ELEN females to 12 TIR females, a significant rare female effect was also detected (P<0.05, Table 1).
To determine the reason for these exceptions, isolation estimates were calculated, using a formula developed by Merrell (1950):
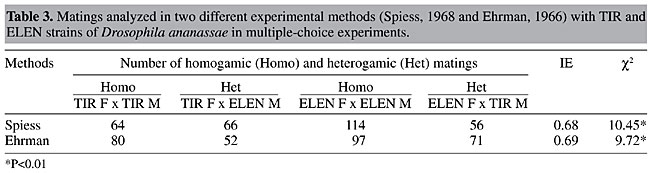
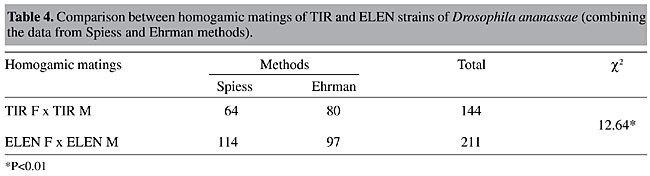
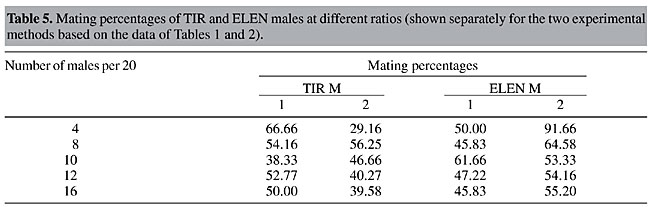
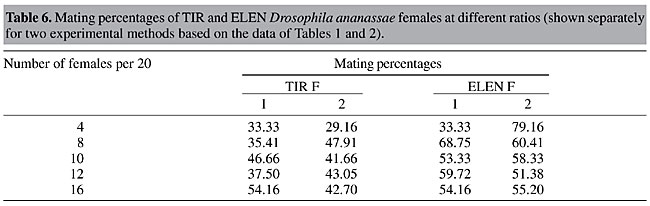
Values of IE were calculated for both experimental methods (Table 3). In both methods, the number of homogamic matings was significantly higher than the number of heterogamic matings (P<0.01), i.e., there was nonrandom, preferential or assortative mating. This is also supported by a slightly lower isolation estimate (0.68-0.69). The degree of preferential mating was higher for ELEN flies than for TIR flies (Table 4, P<0.01). This may be the reason why we found rare male and rare female mating advantages in these isolated cases. The mating percentages of TIR and ELEN males at different ratios (Table 5) were calculated from the data in Tables 1 and 2. Similarly mating percentages of TIR and ELEN females were also determined (Table 6).
DISCUSSION We found no evidence of rare male mating advantage in TIR and ELEN strains of D. ananassae in a previous female-choice technique study (Som and Singh, 2001). The results did not vary between the Spiess and Ehrman methods, nor did two different marking procedures change the outcome. Here, we used multiple-choice experiments and the same two methods to determine mating preference, as well as the same strains of D. ananassae, to study rare male mating advantage. The most accepted explanation for rare male mating advantage is that females discriminate the two types of males present and change their receptivity in favor of minority males (Spiess, 1968; Ehrman and Spiess, 1969; Spiess and Kruckeberg, 1980). Ehrman and Spiess (1969) proposed in their habituation hypothesis that a virgin female at the beginning of a “multiple-choice” experiment gets courtship cues from males and becomes habituated to the majority type of male before her acceptance threshold is reached. Consequently, she would tend to accept a rare type of male, which is able to break through this habituation by presenting slightly different cues and consequently would obtain an advantage in mating. Spiess and Kruckeberg (1980) concluded from their experiments on rare male advantage that it is not the female’s development of sensory habituation to the majority male’s cues but simple avoidance of the first courting male’s cues that accounts for the minority advantage. At unequal ratios first courtship is mostly made by majority males and thus females avoid that cue and accept the rare males. They proposed that the mechanism is a more or less constant avoidance by the female, rather than constant preference, as in O’Donald’s model (1977). Whatever the mechanism, a lot of work has been devoted to unfold the sensory basis of female discrimination. Ehrman (1966, 1972) implicates pheromones as the basis of sexual communication in D. pseudoobscura. Averhoff and Richardson (1974, 1976), who studied the role of pheromones in mating behavior in Drosophila, and Van den Berg et al. (1984) support the habituation hypothesis. In view of this line of reasoning, the absence of rare male mating advantage in our experiments could be due to a lack of a difference in the cues of the males of these two wild type strains of D. ananassae, as a result of which females are unable to discriminate the rare from the common males. Frequently, inconsistencies have been reported in rare male experiments, for example when Partridge and Gardner (1983) repeated the experiments of Spiess and Schwer (1978) and Spiess and Kruckeberg (1980), they did not find any indication of a rare male effect in eye color mutant flies. Interestingly, Peterson and Merrell (1983) found a disadvantage of white eye when these males competed with wild type D. melanogaster. More recently Cakir and Kence (1999) have stated that rare male mating advantage is probably not an important factor for the maintenance of genetic variation in natural populations of Drosophila. Based on the numerous contradictory results it has been postulated that Drosophila mating success is dependent on numerous factors (Spiess, 1970; Parsons, 1973; Knoppien et al., 1980). Actually, a number of factors, e.g., habituation of females to male sexual stimuli, pheromones, auditory signals, genotype fitness, male courtship behavior, male sexual vigor, female age, etc., could result in frequency-dependent mating success in Drosophila, making it difficult to test for rare male mating advantage. In this context, Bryant et al. (1980) have suggested that one should ensure at least some points before starting rare male experiments, such as: competing male types are equally vigorous, marking for identification should have no effect upon mating performance, the number of pairs per strain should be sufficient to override sampling effects and ensure that females (or some of them) do not show a preference for one male type over another. As mentioned earlier, TIR and ELEN strains were equally vigorous and marking for identification had no effect on mating preference. Though the term “vigor” was replaced by “mating propensity” as vigor implies an intrinsic property and has no direct association with mating success. When males differing in mating propensity compete for mates, the advantage for the males with high mating propensity will increase when they are rare because these males compete for mates against other males with high mating propensity when common, but against numerous low-propensity males when they are rare. This may result in one-sided frequency-dependent mating. Therefore, the mating propensity of the strains should initially be more or less equal. According to Bryant et al. (1980), even when two strains have the same sexual vigor, the difference in sexual vigor between sampled lines would not be zero due to sampling effects; consequently, the number of pairs per strain should be large enough to counteract this sampling effect. In our multiple-choice experiments, minority flies were placed in groups of 4 or 8. Thus, there was less chance of sampling error. It is possible that favored rare males gain much of their advantage by mating more than once, which would be revealed by the Ehrman method in which copulating pairs are not aspirated out of the mating chamber. In this case, the Spiess method, in which copulating pairs are removed, would diminish the rare male effect. Also, taking out the flies changes the male ratio, which may affect the result. For this reason, both experimental methods were used. A rare male mating predominance was found in only one isolated case in the flies tested with the Ehrman method (Table 2); this is not sufficient to support the hypothesis of a rare male effect. An advantage for rare females was also found in this same case. There was preferential or assortative mating among the strains (Table 3) as we found a slightly lower isolation estimate; this preferential mating was much greater for ELEN flies (Table 4). This may be the reason for the minority advantage of ELEN flies when 4 pairs were placed with 16 pairs of TIR, and when 8 pairs of ELEN females were used in the Spiess method (see Tables 5 and 6). We could ask, why the same result was not found for ELEN flies in the 4:16 ratio in the Spiess method? In the Ehrman method males could remate, leading to a significant minority advantage that was not possible in the Spiess method. Question may arise, why preferential mating did not lead to rare male mating advantage in all cases? O’Donald (1977) ignored the idea of female discrimination and suggested a constant mating preference of females to a particular male genotype for whom females may have a lower threshold of response. The presence of courting males will raise the general level of stimulation so that all females are stimulated to mate more readily. Thus, a female with a lower threshold towards a particular genotype of male would then mate more readily with any male. The proportion of preferential matings would therefore decline with increasing density. Or it may be that preferential mating prevents minority advantage, as found by Ehrman (1970) among strains of D. paulistorum in which sexual isolation adversely affected minority advantage. Therefore, the lack of evidence for rare male mating advantage in our experiments would not be explained simply by the female discrimination hypothesis; female preference or preferential matings may also play role in these multiple-choice experiments. Interestingly, preferential mating between TIR and ELEN strains has been found using multiple-choice but was not detected in the same strains in female-choice experiments (Som and Singh, 2001). This peculiarity can be attributed to the technique itself. Barker (1962) discussed four methods that are generally used in studies of sexual isolation and selective mating, namely, pair mating or no choice, male choice, female choice and multiple-choice. He and other authors (Merrell, 1949a,b, 1950, 1954, 1960; Casares et al., 1998; Tomaru and Oguma, 2000) have found variation in the degree of isolation determined by different methods. It was found that male- and no choice techniques indicate less isolation between mutant and wild type phenotypes than do female- and multiple-choice experiments (Barker, 1962). In the analysis of selective mating, multiple-choice should be preferred, which gives the closest approach to natural population conditions. In multiple-choice it is possible that sexual activity is increased, as both males and females are present together, compared to female-choice experiments, where females are forced into heterogamic matings, decreasing the opportunity for preferential mating. This may be the reason for the absence of preferential mating in female-choice experiments (Som and Singh, 2001) while it is expressed in the present test using multiple-choice experiments with the same wild type strains. ACKNOWLEDGMENTS Financial assistance in the form of a Research Scholarship from Banaras Hindu University to A. Som is gratefully acknowledged. The authors thank an anonymous reviewer for suggesting corrections in the original draft of the manuscript. REFERENCES Anderson, W.W. and Brown, C.J. (1984). A test for rare male mating advantage with Drosophila pseudoobscura karyotypes. Genetics 107: 577-589. Averhoff, W.W. and Richardson, R.H. (1974). Pheromonal control of mating patterns in Drosophila melanogaster. Behav. Genet. 4: 207-225. Averhoff, W.W. and Richardson, R.H. (1976). Multiple pheromone system controlling mating in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 73: 591-593. Barker, J.S.F. (1962). Studies of selective mating using the yellow mutant of Drosophila melanogaster. Genetics 47: 623-640. Bryant, E.H., Kence, A. and Kimball, K.T. (1980). A rare-male advantage in the housefly induced by wing clipping and some general considerations for Drosophila. Genetics 96: 975-993. Cakir, S. and Kence, A. (1999). Lack of minority advantage in Drosophila melanogaster mutants. Turk. J. Biol. 23: 433-443. Casares, P., Carracedo, M.C., Del Rio, B., Pineiro, R., Garcia-Florez, L. and Barros, A.R. (1998). Disentangaling the effects of mating propensity and mating choice in Drosophila. Evolution 52: 126-133. Dal Molin, C. (1979). An external scent as the basis for a rare-male mating advantage in Drosophila melanogaster. Am. Nat. 113: 951-954. Ehrman, L. (1966). Mating success and genotype frequency in Drosophila. Anim. Behav. 14: 332-339. Ehrman, L. (1968). Frequency dependence of mating success in Drosophila pseudoobscura. Genet. Res. 11: 135-140. Ehrman, L. (1970). Sexual isolation versus mating advantage of rare Drosophila males. Behav. Genet. 1: 111-118. Ehrman, L. (1972). A factor influencing the rare male mating advantage in Drosophila. Behav. Genet. 2: 69-78. Ehrman, L. and Spiess, E.B. (1969). Rare-type mating advantage in Drosophila. Am. Nat. 103: 675-680. Elens, A.A. and Wattiaux, J.M. (1964). Direct observation of sexual isolation. Dros. Inf. Serv. 39: 118-119. Grant, B., Burton, S., Contoreggi, C. and Rothstein, M. (1980). Outbreeding via frequency-dependent mate selection in the parasitoid wasp, Nasonia (= Mormoniella) vitripennis Walker. Evolution 34: 983-992. Joshi, D.S. (1999). Latitudinal variation in locomotor activity rhythm in adult Drosophila ananassae. Can. J. Zool. 77: 865-870. Knoppien, P. (1985a). Rare male mating advantage: a review. Biol. Rev. 60: 81-117. Knoppien, P. (1985b). The numbers of males stored per vial, a possible source of bias in rare male experiments. Dros. Inf. Serv. 61: 101. Knoppien, P., Pot, W. and van Delden, W. (1980). Effects of rearing conditions and age on the difference in mating success between alcohol dehydrogenase genotypes of Drosophila melanogaster. Genetica 51: 197-202. Lewontin, R.C. (1974). The Genetic Basis of Evolutionary Change. Columbia University Press, New York, NY, USA. Markow, T.A. (1980). Rare male advantages among Drosophila of the same laboratory strain. Behav. Genet. 10: 552-556. Markow, T.A. and Smith, L.D. (1979). Genetics of phototactic behavior in Drosophila ananassae, a member of the Drosophila melanogaster species group. Behav. Genet. 9: 61-67. Merrell, D.J. (1949a). Selective mating in Drosophila melanogaster. Genetics 34: 370-389. Merrell, D.J. (1949b). Mating between two strains of Drosophila melanogaster. Evolution 3: 266-268. Merrell, D.J. (1950). Measurement of sexual isolation and selective mating. Evolution 4: 326-331. Merrell, D.J. (1954). Sexual isolation between Drosophila persimilis and Drosophila pseudoobscura. Am. Nat. 88: 93-99. Merrell, D.J. (1960). Mating preferences in Drosophila. Evolution 14: 525-526. O’Donald, P. (1977). Mating advantage of rare males in models of sexual selection. Nature 267: 151-154. Parsons, P.A. (1973). Behavioral and Ecological Genetics: A Study in Drosophila. Clarendon Press, Oxford, England. Partridge, L. and Gardner, A. (1983). Failure to replicate the results of an experiment on the rare male effect in Drosophila melanogaster. Am. Nat. 122: 422-427. Peterson, J.R. and Merrell, D.J. (1983). Rare male mating disadvantage in Drosophila melanogaster. Evolution 37: 1306-1316. Petit, C. (1951). Le role de lisolment sexuel dans levolution des populations de Drosophila melanogaster. Bull. Biol. Fr. Belg. 85: 392-418. Pruzan, A. (1976). Effects of age, rearing and mating experiences on frequency-dependent sexual selection in Drosophila pseudoobscura. Evolution 30: 130-145. Robertson, H.M. (1982). Female courtship summation in Drosophila melanogaster. Anim. Behav. 30: 1105-1117. Singh, B.N. (1985). Drosophila ananassae - a genetically unique species. Nucleus 28: 169-176. Singh, B.N. (1996). Population and behaviour genetics of Drosophila ananassae. Genetica 97: 321-329. Singh, B.N. (2000). Drosophila ananassae: a species characterized by several unusual genetic features. Curr. Sci. 78: 391-398. Singh, B.N. and Chatterjee, S. (1985). Symmetrical and asymmetrical sexual isolation among laboratory strains of Drosophila ananassae. Can. J. Genet. Cytol. 27: 405-409. Singh, B.N. and Chatterjee, S. (1986). Mating ability of homo- and hetero-karyotypes of Drosophila ananassae from natural populations. Heredity 57: 75-78. Singh, B.N. and Chatterjee, S. (1987). Variation in mating propensity and fertility in isofemale strains of Drosophila ananassae. Genetica 73: 237-242. Singh, B.N. and Chatterjee, S. (1988). Selection for high and low mating propensity in Drosophila ananassae. Behav. Genet. 18: 357-370. Singh, B.N. and Chatterjee, S. (1989). Rare-male mating advantage in Drosophila ananassae. Genet. Sel. Evol. 21: 447-455. Singh, B.N. and Pandey, M.B. (1993a). Selection for high and low pupation height in Drosophila ananassae. Behav. Genet. 2: 239-243. Singh, B.N. and Pandey, M.B. (1993b). Evidence for additive polygenic control of pupation height in Drosophila ananassae. Hereditas 119: 111-116. Singh, B.N. and Singh, S.R. (1999). Female remating in Drosophila ananassae: shorter duration of copulation during second mating as compared to first mating. J. Biosci. 24: 427-431. Singh, S.R. and Singh, B.N. (2000). Male remating in Drosophilla ananassae: evidence for interstrain variation in remating time and shorter duration of copulation during second mating. Zool. Sci. 17: 389-393. Singh, B.N. and Sisodia, S. (2000). Frequency-dependent selection: minority male mating advantage in Drosophila. Curr. Sci. 78: 141-150. Som, A. and Singh, B.N. (1998). No effect of marking flies either by nail polish on scutellum or by wing clipping on mating success in Drosophila ananassae. Dros. Inf. Serv. 81: 202-203. Som, A. and Singh, B.N. (2001). Lack of evidence for rare male mating advantage in wild type strains of Drosophila ananassae. Curr. Sci. 81: 383-387. Spiess, E.B. (1968). Low frequency advantage in mating of Drosophila pseudoobscura karyotypes. Am. Nat. 102: 363-379. Spiess, E.B. (1970). Mating propensity and its genetic basis in Drosophila. In: Essays in Evolution and Genetics in Honour of Theodosius Dobzhansky (Hecht, M.K. and Sreere, W.C., eds.). North Holland, Amsterdam, Netherlands, pp. 315-379. Spiess, E.B. and Bowbal, D.A. (1987). Minority mating advantage of certain eye color mutants of Drosophila melanogaster. IV. Female discrimination among three genotypes. Behav. Genet. 17: 291-306. Spiess, E.B. and Kruckeberg, J.F. (1980). Minority advantage of certain eye color mutants of Drosophila melanogaster. II. A behavioral basis. Am. Nat. 115: 307-327. Spiess, E.B. and Schwer, W.A. (1978). Minority mating advantage of certain eye color mutants of Drosophila melanogaster. I. Multiple choice and single female tests. Behav. Genet. 8: 155-168. Spiess, E.B., Langer, B. and Spiess, L.D. (1966). Mating control by gene arrangements in Drosophila pseudoobscura. Genetics 54: 1139-1149. Tobari, Y.N. (Ed.) (1993). Drosophila ananassae. Genetical and Biological Aspects. Japan Scientific Societies Press, Tokyo, Japan. Tomaru, M. and Oguma, Y. (2000). Mate choice in Drosophila melanogaster and D. sechellia: criteria and their variation depending on courtship song. Anim. Behav. 60: 797-804. Van den Berg, M.J., Thomas, G., Hendriks, H. and Van Delden, W. (1984). A re-examination of the negative assortative mating phenomenon and its underlying mechanism in Drosophila melanogaster. Behav. Genet. 14: 45-61. |
|