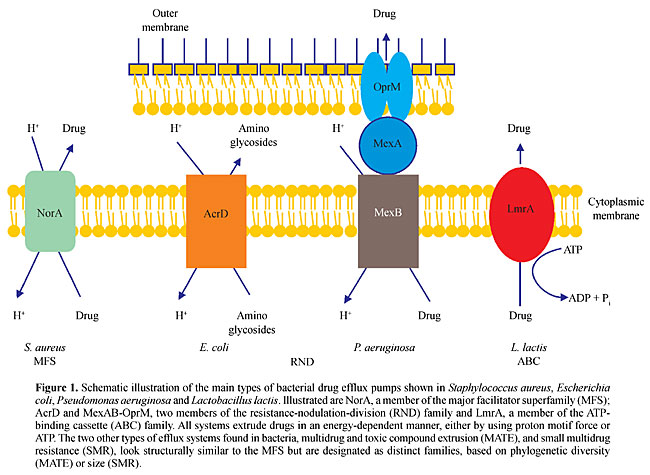
ABSTRACT. Pseudomonas aeruginosa is an opportunistic human pathogen exhibiting innate resistance to multiple antimicrobial agents. This intrinsic multidrug resistance is caused by synergy between a low-permeability outer membrane and expression of a number of broadly-specific multidrug efflux (Mex) systems, including MexAB-OprM and MexXY-OprM. In addition to this intrinsic resistance, these and three additional systems, MexCD-OprJ, MexEF-OprN and MexJK-OprM promote acquired multidrug resistance as a consequence of hyper-expression of the efflux genes by mutational events. In addition to antibiotics, these pumps export biocides, dyes, detergents, metabolic inhibitors, organic solvents and molecules involved in bacterial cell-cell communication. Homologues of the resistance-nodulation-division systems of P. aeruginosa have been found in Burkholderia cepacia, B. pseudomallei, Stenotrophomonas maltophilia, and the nonpathogen P. putida, where they play roles in resistance to antimicrobials and/or organic solvents. Despite intensive studies of these multidrug efflux systems over the past several years, their precise molecular architectures, their modes of regulation of expression and their natural functions remain largely unknown. Key words: Efflux pumps, Antimicrobials, Multidrug resistance, Drug tolerance, Pseudomonas INTRODUCTION The causes for antimicrobial resistance are multifactorial. In the case of antibiotics, it has been well documented that resistance is mainly caused by continued over reliance on and imprudent use of these antibacterial agents (Davies, 1996), and increasing evidence is being obtained suggesting that the same may be true for the emergence of biocide resistance (Russell, 2001; Schweizer, 2001). Of particular concern is the possible cross-resistance of antibiotics and biocides due to common resistance mechanisms (Schweizer, 2001; Levy, 2000, 2002; Poole, 2002). Metal resistance is being observed as the result of polluted environments (Hassan et al., 1999). The consequence of continued exposure to antibacterials is an enrichment of bacteria that are intrinsically resistant to antimicrobials or have acquired resistance mechanisms to these substances (Lerner, 1998; Hellinger, 2000; Livermore, 2000; Poole, 2002). Pseudomonas aeruginosa, Burkholderia ssp. and Stenotrophomonas maltophilia are of clinical significance because of their innate multidrug resistance and their ability to acquire high-level multidrug resistance (Poole, 2001). Although P. putida is not commonly found to be a pathogenic bacterium, it is known for its high-level solvent tolerance. EFFLUX AS A MECHANISM OF ANTIMICROBIAL RESISTANCE Bacterial resistance mechanisms have been mostly determined for antibiotics and include: i) exclusion from the cell, e.g., by the outer membrane of Gram-negative bacteria (Hancock, 1997, 1998); ii) enzymatic inactivation, e.g., by b-lactamases (Thomson and Smith, 2000), by aminoglycoside-modifying enzymes (Davies and Wright, 1997), by chloramphenicol acetyltransferases (Murray and Shaw, 1997), as well as by tetracycline- (Chopra and Roberts, 2001) and by macrolide- (Nakamura et al., 2000) inactivating enzymes; iii) target alterations, e.g., fluoroquinolone resistance (Hooper, 2000) and enterococcal vancomycin resistance (Cetinkaya et al., 2000), and iv) active efflux from the cell, e.g., for fluoroquinolones (Poole, 2000a,b) and for tetracycline (Chopra and Roberts, 2001). Similar resistance mechanisms are also involved in biocide resistance (Russell, 2001; Schweizer, 2001; Levy, 2002; Poole, 2002). Although exclusion from the cell due to reduced outer membrane impermeability was thought to play a key role in the intrinsic resistance of P. aeruginosa and related bacteria to many antimicrobial compounds, this is now attributed to synergy between a low-permeability outer membrane and active efflux from the cell (Poole, 2000a; Poole and Srikumar, 2001). To date, five families of bacterial efflux systems have been identified (Putman et al., 2000). These include: i) the small multidrug resistance family (Chung and Saier Jr., 2001); ii) the resistance-nodulation-division (RND) family (Zgurskaya and Nikaido, 2000), which is part of the larger RND permease superfamily (Tseng et al., 1999); iii) the major facilitator superfamily (Pao et al., 1998); iv) the ATP-binding cassette family (van Veen and Konings, 1998); and v) the multidrug and toxic compound extrusion family (Brown et al., 1999). Well-characterized representatives of these families from Gram-positive and Gram-negative bacteria are shown in Figure 1. All of these transporters catalyze active drug efflux and therefore require energy, mostly in the form of proton motif force, but some also in the form of ATP (Paulsen et al., 1996). In P. aeruginosa and related bacteria, the RND family is the best characterized (Poole, 2001) and is of clinical significance (Poole and Srikumar, 2001), and therefore this review focuses on this important family of efflux pumps. The prevalence of RND pumps in bacteria varies widely, ranging from none in Mycobacterium tuberculosis, 1 in Bacillus subtilis, 4 in Escherichia coli to 12 in P. aeruginosa (Stover et al., 2000).
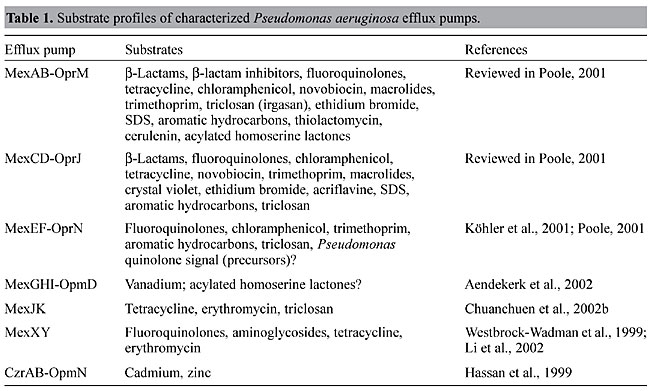
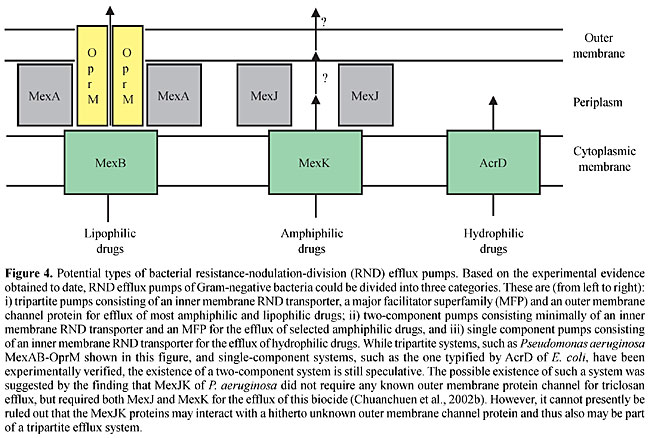
ARCHITECTURE OF RND EFFLUX PUMPS Most lipophilic and amphiphilic drugs can cross the cytoplasmic membrane spontaneously, and their accumulation in the periplasm would accelerate their reentry into the cytoplasm. To take full advantage of the above-mentioned exclusion properties of the cell wall in these bacteria, and to bypass the periplasm, it has therefore been proposed that tripartite efflux systems, consisting of a cytoplasmic membrane-associated drug-proton antiporter of the RND family, a periplasmic membrane fusion protein (MFP) and an outer membrane channel-forming protein are required to expel drugs from the cytoplasm to the surrounding medium (Zgurskaya and Nikaido, 1999a,b, 2000). However, variants of this tripartite system have been observed. The AcrD system of E. coli consists of only the RND-type cytoplasmic membrane-associated transporter involved in efflux of aminoglycosides (Rosenberg et al., 2000). The existence of a single-component RND transporter for aminoglycosides can be rationalized because the main permeability barrier for hydrophilic drugs, like aminoglycosides, is the cytoplasmic membrane and therefore expulsion into the periplasm achieves its purpose. Although the exact molecular architecture of a tripartite RND efflux system still remains a mystery, some recent progress has been made regarding the topologies of the outer membrane protein component, OprM, and the RND transporter component, MexA, of one P. aeruginosa efflux pump, MexAB-OprM. Functional OprM is a trimeric protein, which besides the typical barrel configuration of the outer membrane-embedded portion of the protein exhibits long periplasmic extensions capable of spanning the periplasm (Wong et al., 2001). This led to the proposal that OprM is capable of directly interacting with the RND transporter, MexB. A topology analysis of MexB using alkaline phosphatase fusions not only predicted 12 transmembrane domains typical of such transporter proteins, but also several extensive periplasmic loops (Guan et al., 1999). Therefore, it is conceivable that OprM directly interacts with MexB. What role the MFP MexA plays in this process remains a mystery although it is clear that it is required for the functioning of RND efflux pumps (Zgurskaya and Nikaido, 1999a,b). RND EFFLUX PUMPS OF PSEUDOMONAS AERUGINOSA An analysis of the genome sequence of this bacterium reveals the existence of 12 potential RND efflux systems (Figure 2) (Stover et al., 2000). Of these, only the function of six - MexAB-OprM (Poole et al., 1993), MexCD-OprJ (Poole et al., 1996), MexEF-OprN (Köhler et al., 1997), MexGHI-OpmD (Aendekerk et al., 2002), MexJK (Chuanchuen et al., 2002b) and MexXY (Mine et al., 1999; Westbrock-Wadman et al., 1999) - have been experimentally confirmed. Known substrates for these pumps are listed in Table 1. From these listings it is clear that although the substrate spectrum for many of these pumps is wide, some selectivity exists and, perhaps not too surprising, it rests with the RND transporters of the Mex pumps (Srikumar et al., 1997; Maseda et al., 2000b).
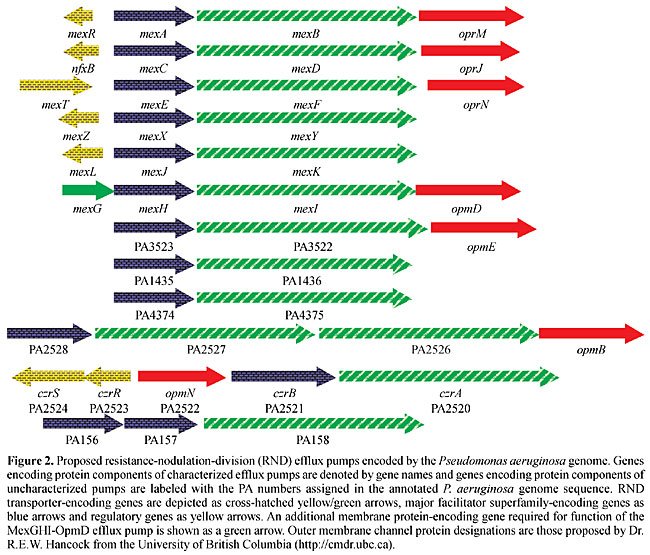
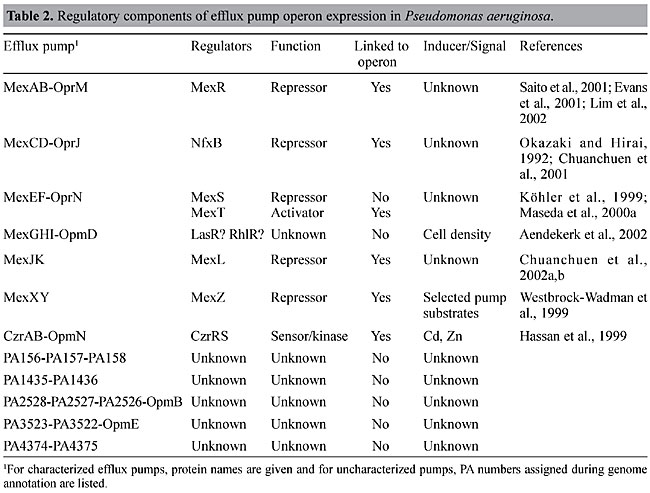
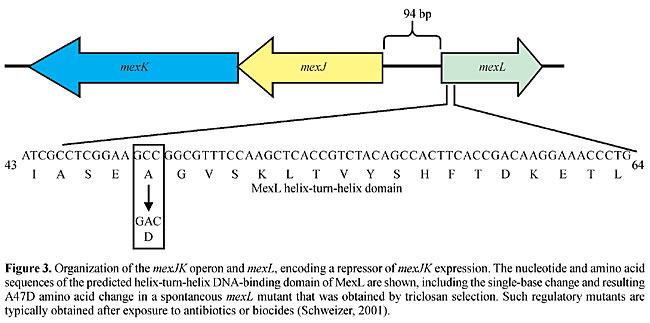
The genetic organization of the P. aeruginosa RND efflux operons (Figure 2) reveals several commonalities, as well as some significant differences. First, genes encoding the RND transporter and corresponding MFP, respectively, are always present. Second, genes encoding regulators and outer membrane channel proteins, respectively, are not always present, and, in fact, some operons neither contain a regulatory gene nor an outer membrane protein-encoding gene linked to the efflux operon. Third, some operons contain additional genes besides those encoding the RND transporter and MFP, respectively. For example, mexGHI-opmD contains mexG, which encodes a membrane protein that is required for pump function (Aendekerk et al., 2002). Similarly, PA2528-PA2527-PA2526-opmB (Figure 2) contains an additional RND transporter-encoding gene, PA2526. Several questions arise from these observations: i) does the absence of a regulatory protein equate with constitutive expression of the respective operon? and ii) do pumps always require an outer membrane protein channel for function and, if so, which outer membrane channel protein(s) do they use? REGULATION OF EXPRESSION OF PSEUDOMONAS AERUGINOSA RND EFFLUX PUMPS Table 2 summarizes what we know to date about regulators and inducers of P. aeruginosa efflux pumps. With the exception of MexAB-OprM, the expression of most of these efflux systems is tightly regulated and this has recently been verified by transcriptional profiling using Affymetrix Gene Chips® (Lory, S., personal communication; Chuanchuen, R. and Schweizer, H.P., unpublished results). To date, the expression of only one RND system, MexXY, has been shown to be inducible in the presence of selected antibiotics (Masuda et al., 2000b) and expression of the MexGHI-OpmD pump is cell density dependent, i.e., regulated by the quorum-sensing regulatory circuit(s) (Aendekerk et al., 2002). Overexpression of the other RND pumps is observed as a consequence of exposure to antimicrobials in vivo and in vitro and this overexpression is usually caused by mutations in genes encoding regulatory proteins (Poole et al., 1996; Saito et al., 1999; Westbrock-Wadman et al., 1999; Ziha-Zafiri et al., 1999; Jalal et al., 1999, 2000; Maseda et al., 2000a; Join-Lambert et al., 2001; Chuanchuen et al., 2002b). These mutations are stable, since expression persists long after removal of selective pressure. For example, triclosan-resistant derivatives of a triclosan-susceptible P. aeruginosa mutant strain expressed the MexJK efflux pump due to a mutation in the cognate regulatory gene, mexL (Chuanchuen et al., 2000a,b). This single-base change led to an alanine to an aspartic acid change in the second helix of the putative helix-turn-helix putative DNA-binding domain of MexL (Figure 3). Therefore, this mutation was predicted to result in the production of an inactive MexL protein and, in fact, when the binding activity of purified MexL mutant protein was compared to that of the wild-type MexL protein it was unable to bind to its target DNA (Chuanchuen and Schweizer, 2002b). The hitherto best characterized mex regulatory protein is MexR, a regulator of MexAB-OprM expression. Although the mexAB-oprM operon is always transcribed at low but detectable levels, MexAB-OprM is overproduced in MexR mutants (Saito et al., 1999; Adewoye et al., 2002). The MexR protein was purified, its binding characteristics were characterized (Evans et al., 2001; Saito et al., 2001), and its structure with the operator sites was determined (Lim et al., 2002). The other known regulatory genes (Table 2), nfxB (Okazaki and Hirai, 1992; Shiba et al., 1995; Poole et al., 1996), mexZ (or amrR) (Westbrock-Wadman et al., 1999) and mexL (Chuanchuen et al., 2002b) encode negative regulators, and mutations in these genes lead to overexpression of the mexAB-oprM, mexCD-oprJ, mexXY and mexJK operons, respectively. NfxB and MexL have also been purified and shown to bind to DNA upstream of mexC (Shiba et al., 1995) and mexJ (Chuanchuen et al., 2002a), respectively. The mexEF-oprN operon is positively regulated by the mexT product, encoding a transcriptional activator of the LysR family (Köhler et al., 1999). Interestingly, with the exception of the Geneva type strain, which is mexT +, most PAO1 wild-type strains and their derivatives contain mexT mutations preventing the expression of MexEF-OprN in these strains in the absence of mexT reversions or suppressor mutations (Maseda et al., 2000a; Köhler et al., 2001).
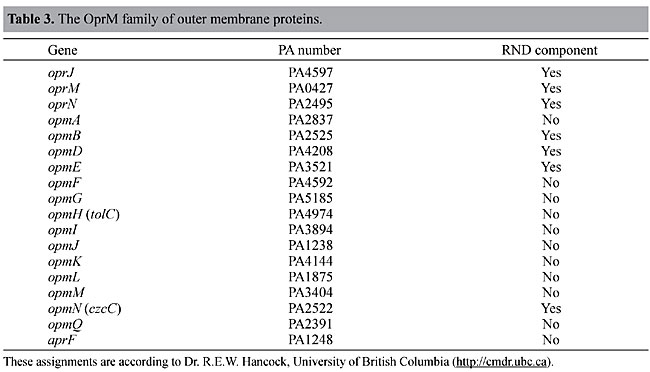
Besides these known regulators, other negative regulatory factors must also be involved in efflux operon expression. Many clinical isolates constitutively express various efflux pumps in the absence of identifiable regulatory mutations, suggesting regulation by hitherto unidentified regulators (Ziha-Zafiri et al., 1999; Pumbwe and Piddock, 2000; Beinlich et al., 2001). Other regulatory factors are further suggested since most of the efflux systems of unknown function are tightly regulated, although they do not contain regulatory genes in the vicinity of their structural genes (Figure 2). To date, few inducers for any of the RND multidrug efflux operons have been identified. The CzrAB-OpmN divalent cation RND efflux pump (Hassan et al., 1999) is regulated by a two-component regulatory system in the presence of divalent cations. Some clinical isolates resisting antibiotic treatment in patients are susceptible to the same antibiotics when isolated and propagated in the laboratory, suggesting inducible components of resistance. It is not known whether this is due to inducible efflux pumps or other mechanisms. Efflux pumps may be involved because MexXY expression is induced in laboratory cultures in the presence of selected pump substrates but this “induction” is not mediated via mexZ (Masuda et al., 2000a). Clearly, much work has yet to be done to understand the regulatory circuits underlying RND efflux pump expression in P. aeruginosa. OUTER MEMBRANE CHANNEL REQUIREMENT Since Gram-negative bacteria possess two membranes separated by the intervening periplasmic space, it is now generally assumed that RND efflux systems in these bacteria function as tripartite systems, which enable the bacterium to extrude antibacterials from the cytoplasm all the way to the extracellular medium (Zgurskaya and Nikaido, 2000). The evidence that even RND systems missing their own outer membrane channels nonetheless require such a protein to function as an efflux pump solely rests on the observations that MexXY (Masuda et al., 2000a; Chuanchuen et al., 2001) and MexJK (Chuanchuen et al., 2002b) require OprM in order to form a functional pump for the export of antibiotics. OprM was proposed because it is always expressed at low but detectable levels from a secondary promoter independent of MexAB (Zhao et al., 1998). However, the identity of the cognate outer membrane channel components, if any, and their interchangeability among different efflux pumps remains under considerable debate. While OprM can function with all RND transporter/MFP complexes studied to date, including MexCD (Gotoh et al., 1998), MexEF (Maseda et al., 2000b), MexXY (Mine et al., 1999; Masuda et al., 2000a; Chuanchuen et al., 2001) and MexJK (Chuanchuen et al., 2002b), other outer membrane channel proteins are more discriminative. Whereas OprJ and OprN did not function with MexJK (Chuanchuen et al., 2002b), and OprN failed to interact with MexAB (Maseda et al., 2000b), OprJ restored almost complete function to a MexAB system without OprM (Srikumar et al., 1997). Further complicating the picture significantly is the fact that the OprM family of outer membrane proteins contains 18 members (Table 3), of which only 7 are associated with RND efflux pumps and we know very little about the functionality, if any, of these proposed outer membrane protein channels. Although MexJK requires OprM for the export of antibiotics and dyes, it seems capable of promoting efflux of the common biocide triclosan, as a two-component system, consisting only of the cytoplasmic membrane-associated MexK RND transporter and the periplasmic MexJ MFP (Chuanchuen et al., 2002b). The question therefore is whether it is possible that RND pumps sometimes function as two-component pumps for the efflux of some substrates, possibly their natural substrates. This may also explain why 6 of 12 RND pumps did not maintain a gene encoding their own outer membrane channel proteins during evolution (Figure 2). From our current knowledge, we cannot rule out the possibility that a two-component RND efflux system is sufficient for the efflux of selected amphiphilic drugs, such as triclosan. If this were the case, then RND efflux pumps could be divided into three categories: i) single-component pumps consisting of an inner membrane RND transporter, as typified by AcrD of E. coli, for the efflux of hydrophilic drugs; ii) two-component pumps, consisting minimally of an inner membrane RND transporter and an MFP for the efflux of selected amphiphilic drugs, and iii) tripartite pumps consisting of an inner membrane RND transporter, an MFP and an outer membrane protein channel for the efflux of most lipophilic drugs (Figure 4).
PHYSIOLOGICAL ROLE OF RND EFFLUX SYSTEMS IN PSEUDOMONAS AERUGINOSA Despite rather intense investigations over the last few years, the physiological role(s) of the RND efflux pumps in P. aeruginosa remain largely unclear, with the exception of MexGHI-OpmD and CzrAB-OpmN, which function as metal efflux systems (Hassan et al., 1999; Aendekerk et al., 2002). Although RND pumps could function as efflux systems for undesirable metabolic by-products, they may also play a more active role in cellular metabolism. For example, strains hyperexpressing and/or lacking MexAB-OprM, MexEF-OprN and MexGHI-OpmD either exhibit altered levels of extracellular acylated homoserine lactones or other phenotypes tied to quorum sensing (Evans et al., 1998; Köhler et al., 2001; Aendekerk et al., 2002). Therefore, these systems may play a role in the efflux of quorum-sensing molecules or their metabolic precursors and thus play an important role in quorum-sensing signal homeostasis. However, more research is needed to establish a firm link between the expression of efflux pumps and bacterial quorum sensing. EFFLUX SYSTEMS IN OTHER BACTERIA RND efflux systems are also found in bacteria that exhibit cell wall properties similar to P. aeruginosa (Poole, 2001). Although originally identified as a plant pathogenic bacterium, Burkholderia cepacia is a human pathogen of increasing importance (Govan and Deretic, 1996). It is resistant to multiple antimicrobial agents (Quinn, 1998) and this resistance can be in large part attributed to the expression of the CeoAB-OpcM efflux pump, which confers resistance to chloramphenicol, trimethoprim and fluoroquinolones (Burns et al., 1996). The expression of CeoAB-OpcM is regulated by salicylate, presumably because it mimics iron starvation conditions (Burns and Clark, 1992). Burkholderia pseudomallei is the causative agent of meliodosis (Dance, 1991) and is highly antibiotic resistant (Simpson et al., 1999). The AmrAB-OprA RND efflux pump confers resistance to aminoglycosides and macrolides, and a D(amrAB-oprA) mutant became highly susceptible to multiple antibiotics (Moore et al., 1999). The expression of this efflux pump is governed by the adjacent regulatory gene AmrR (Moore et al., 1999). Stenotrophomonas maltophilia is an increasingly important nosocomial pathogen in debilitated and immunosuppressed individuals (Quinn, 1998; Valdezate et al., 2001). Like P. aeruginosa and Burkholderia ssp., it is also resistant to multiple antimicrobial agents (Denton and Kerr, 1998; Quinn, 1998). Thus far, two RND efflux systems have been identified, SmeABC (Li et al., 2002) and SmeDEF (Alonso and Martinez, 2000; Zhang et al., 2001), and SmeDEF was shown to be expressed in clinical isolates (Alonso and Martinez, 2001). Expression of SmeABC is probably positively regulated by the SmeRS two-component regulatory system, whose structural genes are located upstream of the smeABC operon (Li et al., 2002). Although P. putida is rarely a pathogen, it is known for its high solvent tolerance (Inoue and Horikoshi, 1989) and this is in great part attributable to active efflux (Isken and de Bont, 1996). The responsible efflux systems may or may not efflux medically relevant antimicrobials. SrpABC from strain S12 (Isken and de Bont, 1998; Kieboom et al., 1998a) and TgtDEF from strain DOT-T1E confer toluene tolerance but not resistance to medically relevant antimicrobials (Mosqueda and Ramos, 2000). In contrast, MepABC from strain KT2442 (Fukumori et al., 1998) and ttgABC from strain DOT-1E (Ramos et al., 1998) efflux solvents and antimicrobials. Since the expression of TtgABC (Ramos et al., 1998), TtgDEF (Mosqueda and Ramos, 2000) and SrpABC (Kieboom et al., 1998b) is inducible by aromatic hydrocarbons, the primary physiological role of most of these efflux systems is probably to confer solvent tolerance. CONCLUDING REMARKS A major factor governing antimicrobial resistance in P. aeruginosa and related bacteria is synergy between reduced outer membrane permeability and active efflux from the cell via RND efflux pumps. In pathogenic bacteria these pumps are of clinical significance since they are expressed in clinical bacterial isolates and most of them are involved in the extrusion of clinically significant antibiotics. Although RND pumps have been studied in some detail, much research remains to be performed, particularly to advance our understanding of their regulation of expression, of their outer membrane channel protein requirements and of their physiological function. Most of our knowledge about RND efflux pump expression and function stems from work that has been performed with E. coli and P. aeruginosa, but efflux pumps in other medically, agriculturally and medically important bacteria are not yet well understood. The finding that P. aeruginosa mutants that express no efflux pumps are susceptible to most clinically useful antibiotics, including fluoroquinolones, b-lactams and aminoglycosides, prompted research into efflux pump inhibitors, and several such inhibitors have been identified (Renau et al., 1999; Lomovskaya et al., 2001). Some of these are broad-spectrum RND pump inhibitors, while others inhibit individual efflux pumps. Inhibition of efflux pumps significantly decreased the level of intrinsic resistance, reversed acquired resistance and resulted in decreased frequency of emergence of resistance to efflux pump substrates (Lomovskaya et al., 2001). Inhibition of efflux pumps is therefore an attractive approach to improve the efficacies of antibiotics that are substrates of these pumps. ACKNOWLEDGMENTS Work in the author’s laboratory was supported by grant GM56685 from the National Institutes of Health and grants from the College Research Council of the College of Veterinary Medicine and Biomedical Sciences at Colorado State University. I also acknowledge all of the people who contributed to this work, especially Rungtip “Jeed” Chuanchuen. REFERENCES Adewoye, L., Sutherland, A., Srikumar, R. and Poole, K. (2002). The MexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutants compromising activity. J. Bacteriol. 184: 4308-4312. Aendekerk, S., Ghysels, B., Cornelis, P. and Baysse, C. (2002). Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology 148: 2371-2381. Alonso, A. and Martinez, J.L. (2000). Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 44: 3079-3086. Alonso, A. and Martinez, J.L. (2001). Expression of multidrug efflux pump SmeDEF by clinical isolates of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 45: 1879-1881. Beinlich, K.L., Chuanchuen, R. and Schweizer, H.P. (2001). Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 198: 129-134. Brown, M.H., Paulsen, I.T. and Skurray, R.A. (1999). The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31: 393-395. Burns, J.L. and Clark, D.K. (1992). Salicylate-inducible resistance in Pseudomonas cepacia associated with absence of a pore-forming outer membrane protein. Antimicrob. Agents Chemother. 36: 2280-2285. Burns, J.L., Wadsworth, C.D., Barry, J.J. and Goodall, C.P. (1996). Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob. Agents Chemother. 40: 307-313. Cetinkaya, Y., Falk, P. and Mayhall, C.G. (2000). Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13: 686-707. Chopra, I. and Roberts, M. (2001). Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65: 232-260. Chuanchuen, R., Beinlich, K., Hoang, T.T., Becher, A., Karkhoff-Schweizer, R.R. and Schweizer, H.P. (2001). Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45: 428-432. Chuanchuen, R., Narasaki, C.T. and Schweizer, H.P. (2002a). Molecular characterization of MexL, a repressor of the MexJK multidrug efflux system-encoding operon of Pseudomonas aeruginosa. Abstracts of the 102nd General Meeting of the American Society for Microbiology: 168. Chuanchuen, R., Narasaki, C.T. and Schweizer, H.P. (2002b). The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184: 5036-5044. Chung, Y.J. and Saier Jr., M.H. (2001). SMR-type multidrug resistance pumps. Curr. Opin. Drug Disc. Dev. 4: 237-245. Dance, D.A.B. (1991). Meliodosis: the tip of the iceberg? Clin. Microbiol. Rev. 4: 52-60. Davies, J. (1996). Origins and evolution of antibiotic resistance. Microbiologia 12: 9-16. Davies, J. and Wright, G.D. (1997). Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5: 234-240. Denton, M. and Kerr, K.G. (1998). Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11: 57-80. Evans, K., Passador, L., Srikumar, R., Tsang, E., Nezezon, J. and Poole, K. (1998). Influence of the MexAB-OprM efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 180: 5443-5447. Evans, K., Adewoye, L. and Poole, K. (2001). MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR intergenic region. J. Bacteriol. 183: 807-812. Fukumori, F., Hirayama, H., Takami, H., Inoue, A. and Horikoshi, K. (1998). Isolation and transposon mutagenesis of a Pseudomonas putida KT2442 toluene-resistant variant: involvement of an efflux system in solvent resistance. Extremophiles 2: 395-400. Gotoh, N., Tsujimoto, H., Nomura, A., Okamoto, K., Tsuda, M. and Nishino, T. (1998). Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 165: 21-27. Govan, J.R.W. and Deretic, V. (1996). Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60: 539-574. Guan, L., Ehrmann, M., Yoneyama, H. and Nakae, T. (1999). Membrane topology of the xenobiotic-exporting subunit, MexB, of the MexA,B-OprM extrusion pump in Pseudomonas aeruginosa. J. Biol. Chem. 274: 10517-10522. Hancock, R.E.W. (1997). The bacterial outer membrane as a drug barrier. Trends Microbiol. 5: 37-42. Hancock, R.E.W. (1998). Resistance mechanisms in Pseudomonas aeruginosa and other non-fermentative bacteria. Clin. Infect. Dis. 27 (Suppl. 1): S93-S99. Hassan, M.-E.-T., van der Lelie, D., Springael, D., Römling, U., Ahmed, N. and Mergeay, M. (1999). Identification of a gene cluster, czr, involved in cadmium and zinc resistance in Pseudomonas aeruginosa. Gene 238: 417-425. Hellinger, W.C. (2000). Confronting the problem of increasing antibiotic resistance. South. Med. J. 93: 842-848. Hooper, D.C. (2000). Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 31 (Suppl. 2): S24-S28. Inoue, A. and Horikoshi, K. (1989). A Pseudomonas thrives in high concentration of toluene. Nature 338: 264-265. Isken, S. and de Bont, J.A.M. (1996). Active efflux of toluene in a solvent-resistant bacterium. J. Bacteriol. 178: 6056-6058. Isken, S. and de Bont, J.A.M. (1998). Bacteria tolerant to organic solvents. Extremophiles 3: 229-238. Jalal, S., Wretlind, G., Gotoh, N. and Wretlind, B. (1999). Rapid identification of mutations in a multidrug efflux pump in Pseudomonas aeruginosa. Acta Pathol. Microbiol. Immunol. Scand. 107: 1109-1116. Jalal, S., Ciofu, O., Hoiby, N., Gotoh, N. and Wretlind, B. (2000). Molecular mechanisms of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 44: 710-712. Join-Lambert, O.F., Michea-Hamzehpour, M., Köhler, T., Chau, F., Faurisson, F., Dautrey, S., Vissuzaine, C., Carbon, C. and Pechere, J.-C. (2001). Differential selection of multidrug efflux mutants by trovafloxacin and ciprofloxacin in an experimental model of Pseudomonas aeruginosa acute pneumonia in rats. Antimicrob. Agents Chemother. 45: 571-576. Kieboom, J., Dennis, J.J., de Bont, J.A. and Zylstra, G.J. (1998a). Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J. Biol. Chem. 273: 85-91. Kieboom, J., Dennis, J.J., Zylstra, G.J. and de Bont, J.A. (1998b). Active efflux of organic solvents by Pseudomonas putida S12 is induced by solvents. J. Bacteriol. 180: 6769-6772. Köhler, T., Michea-Hamzehpour, M., Henze, U., Gotoh, N., Curty, L.K. and Pechere, J.C. (1997). Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23: 345-354. Köhler, T., Epp, S.F., Curty, L.K. and Pechere, J.-C. (1999). Characterization of MexT, the regulator of the mexE-mexF-oprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181: 6300-6305. Köhler, T., van Delden, C., Kocjanic Curty, L., Hamzehpour, M.M. and Pechere, J.-C. (2001). Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183: 5213-5222. Lerner, S.A. (1998). Clinical impact of antibiotic resistance. Adv. Exp. Med. Biol. 456: 7-15. Levy, S.B. (2000). Antibiotic and antiseptic resistance: impact on Public Health. Ped. Infect. Dis. J. 19: S120-S122. Levy, S.B. (2002). Active efflux, a common resistance mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 92: 65S-71S. Li, X.-Z., Zhang, L. and Poole, K. (2002). SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 46: 333-343. Lim, D., Poole, K. and Strynadka, N. (2002). Crystal structure of the MexR repressor of the mexRABM-oprM multi-drug efflux operon of Pseudomonas aeruginosa. J. Biol. Chem. 277: 29253-29259. Livermore, D.M. (2000). Epidemiology of antibiotic resistance. Intensive Care Med. 26 (Suppl. 1): S14-S21. Lomovskaya, O., Warren, M.S., Lee, A., Galazzo, J., Fronko, R., Lee, M., Blais, J., Cho, D., Chamberland, S., Renau, T., Leger, R., Hecker, S., Watkins, W., Hoshino, K., Ishida, H. and Lee, V.J. (2001). Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45: 105-116. Maseda, H., Saito, K., Nakajima, A. and Nakae, T. (2000a). Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 192: 107-112. Maseda, H., Yoneyama, H. and Nakae, T. (2000b). Assignment of the substrate-selective subunits of the MexEF-OprN multidrug efflux pump of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44: 658-664. Masuda, N., Sagagawa, E., Ohya, S., Gotoh, N., Tsujimoto, H. and Nishino, T. (2000a). Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44: 2242-2246. Masuda, N., Sakagawa, E., Ohya, S., Gotoh, N., Tsujimoto, H. and Nishino, T. (2000b). Substrate specificities of MexAB-OprM, MexCD-OprJ and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44: 3322-3327. Mine, T., Morita, Y., Kataoka, A., Mizushima, T. and Tsuchiya, T. (1999). Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43: 415-417. Moore, R.A., DeShazer, D., Reckseidler, S., Weissman, A. and Woods, D.E. (1999). Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43: 465-470. Mosqueda, G. and Ramos, J.L. (2000). A set of genes encoding a second toluene efflux system from Pseudomonas putida DOT-1E is linked to the tod genes for toluene metabolism. J. Bacteriol. 182: 937-943. Murray, I.A. and Shaw, W.V. (1997). O-acetyltransferases for chloramphenicol and other natural products. Antimicrob. Agents Chemother. 41: 1-6. Nakamura, A., Nakazawa, K., Miyakozawa, I., Mizukoshi, S., Tsurubuchi, K., Nakagawa, M., O’Hara, K. and Sawai, T. (2000). Macrolide esterase-producing Escherichia coli clinically isolated in Japan. J. Antibiot. 53: 516-624. Okazaki, T. and Hirai, K. (1992). Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol. Lett. 97: 197-202. Pao, S.S., Paulsen, I.T. and Saier Jr., M.H. (1998). Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62: 1-34. Paulsen, I.T., Brown, M.H. and Skurray, R.A. (1996). Proton-dependent multidrug efflux systems. Microbiol. Rev. 60: 575-608. Poole, K. (2000a). Efflux-mediated resistance to fluoroquinolones in Gram-negative bacteria. Antimicrob. Agents Chemother. 44: 2233-2241. Poole, K. (2000b). Efflux-mediated resistance to fluoroquinolones in Gram-positive bacteria and the mycobacteria. Antimicrob. Agents Chemother. 44: 2595-2599. Poole, K. (2001). Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3: 255-264. Poole, K. (2002). Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 92: 55S-64S. Poole, K. and Srikumar, R. (2001). Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr. Topics Med. Chem. 1: 59-71. Poole, K., Krebes, K., McNally, C. and Neshat, S. (1993). Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175: 7363-7372. Poole, K., Gotoh, N., Tsujimoto, H., Zhao, Q., Wada, A., Yamasaki, T., Neshat, S., Yamagishi, J., Li, X.Z. and Nishino, T. (1996). Overexpression of the mexC-mexD-oprJ effux operon in nfxB-type multidrug resistant strains. Mol. Microbiol. 21: 713-724. Pumbwe, L. and Piddock, L.J. (2000). Two efflux systems expressed simultaneously in multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44: 2861-2864. Putman, M., van Veen, H.W. and Konings, W.N. (2000). Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64: 672-693. Quinn, J.P. (1998). Clinical problems posed by multiresistant nonfermenting Gram-negative pathogens. Clin. Infect. Dis. 27 (Suppl. 1): S117-S124. Ramos, J.L., Duque, E., Godoy, P. and Segura, A. (1998). Efflux pumps involved in toluene tolerance of Pseudomonas putida DOT-1E. J. Bacteriol. 180: 3323-3329. Renau, T.E., Leger, R., Flamme, E.M., Sangalang, J., She, M.W., Yen, R., Gannon, C.L., Griffith, D., Chamberland, S., Lomovskaya, O., Hecker, S.J., Lee, V.J., Ohta, T. and Nakayama, K. (1999). Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42: 4928-4931. Rosenberg, E.Y., Ma, D. and Nikaido, H. (2000). AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182: 1754-1756. Russell, A.D. (2001). Mechanisms of bacterial insusceptibility to biocides. Am. J. Infect. Control 29: 259-261. Saito, K., Yoneyama, H. and Nakae, T. (1999). nalB-type mutations causing the overexpression of the MexAB-OprM efflux pump are located in the mexR gene of the Pseudomonas aeruginosa chromosome. FEMS Microbiol. Lett. 179: 67-72. Saito, K., Eda, S., Maseda, H. and Nakae, T. (2001). Molecular mechanism of MexR-mediated regulation of MexAB-OprM efflux pump expression in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 195: 23-28. Schweizer, H.P. (2001). Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 202: 1-7. Shiba, T., Ihiguro, K., Takemoto, N., Koibuchi, H. and Sugimoto, K. (1995). Purification and characterization of the P. aeruginosa NfxB protein, the negative regulator of the nfxB gene. J. Bacteriol. 177: 5872-5877. Simpson, A.J., White, N.J. and Wuthiekanun, V. (1999). Aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43: 2332 (Letter to the Editor). Srikumar, R., Li, X.-Z. and Poole, K. (1997). Inner membrane efflux components are responsible for the b-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J. Bacteriol. 179: 7875-7881. Stover, C.K., Pham, X.-Q., Erwin, A.L., Mizoguchi, S.D., Warrener, P., Hickey, M.J., Brinkman, F.S.L., Hufnagle, W.O., Kowalik, D.J., Lagrou, M., Garber, R.L., Goltry, L., Tolentino, E., Westbrock-Wadman, S., Yuan, Y., Brody, L.L., Coulter, S.N., Folger, K.R., Kas, A., Larbig, K., Lim, R., Spencer, D., Wong, G.K.-S., Wu, Z., Paulsen, I.T., Reizer, J., Saier, M.H., Hancock, R.E.W., Lory, S. and Olson, M.V. (2000). Complete genome sequence of Pseudomonas aeruginosa, an opportunistic pathogen. Nature 406: 959-964. Thomson, K.S. and Smith, M.E. (2000). Version 2000: the new beta-lactamases of gram-negative bacteria at the dawn of the new millenium. Microbes Infect. 2: 1225-1235. Tseng, T.-T., Gratwick, K.S., Kollman, J., Park, D., Nies, D.H., Goffeau, A. and Saier Jr., M.H. (1999). The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1: 107-125. Valdezate, S., Vindel, A., Maiz, L., Baquero, F., Escobar, H. and Canton, R. (2001). Persistence and variability of Stenotrophomonas maltophilia in cystic fibrosis patients. Emerging Infect. Dis. 7: 113-122. van Veen, H.W. and Konings, W.N. (1998). The ABC family of multidrug transporters in microorganisms. Biochim. Biophys. Acta 1365: 31-36. Westbrock-Wadman, S., Sherman, D.R., Hickey, M.J., Coulter, S.N., Zhu, Y.Q., Warrener, P., Nguyen, L.Y., Shawar, R.M., Folger, K.R. and Stover, C.K. (1999). Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside resistance. Antimicrob. Agents Chemother. 43: 2975-2983. Wong, K.K.Y., Brinkman, F.S.L., Benz, R.S. and Hancock, R.E.W. (2001). Evaluation of a structural model of Pseudomonas aeruginosa outer membrane protein OprM, an efflux component involved in intrinsic antibiotic resistance. J. Bacteriol. 183: 367-374. Zgurskaya, H.I. and Nikaido, H. (1999a). AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285: 409-420. Zgurskaya, H.I. and Nikaido, H. (1999b). Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA 96: 7190-7195. Zgurskaya, H.L. and Nikaido, H. (2000). Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37: 219-225. Zhang, L., Li, X.-Z. and Poole, K. (2001). SmeDEF multidrug efflux pump contributes to intrinsic multidrug resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 45: 3497-3503. Zhao, Q., Li, X.Z., Srikumar, R. and Poole, K. (1998). Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob. Agents Chemother. 42: 1682-1688. Ziha-Zafiri, I., Llanes, C., Köhler, T., Pechere, J.-C. and Plesiat, P. (1999). In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 43: 287-291. |
|