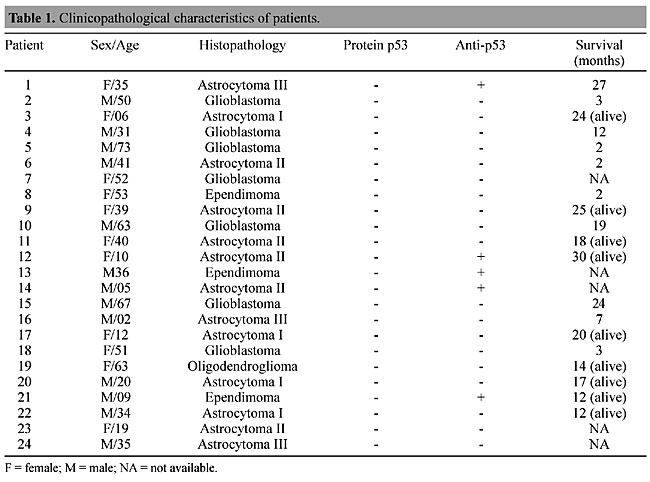
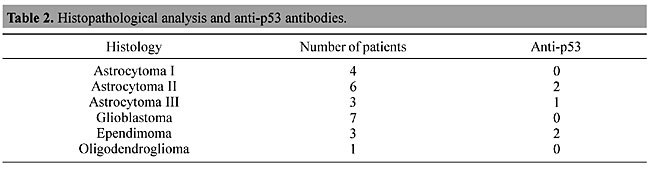
ABSTRACT. Gliomas of astrocytic origin are the most common primary brain tumors, accounting for over 40 to 50% of all central nervous system tumors. The TP53 tumor suppressor gene is the most frequently mutated gene found in human malignancies. A mutation of this gene can lead to an increased half-life of the resulting protein and loss of biological function. High levels of p53 have been detected in the serum of colon cancer patients, although p53 protein has not been detected in the serum of brain tumor patients. Besides circulating p53, several studies have detected antibodies against p53 in patients with lung and breast cancer, as well as those with other types of cancer. We studied p53 protein and anti-p53 antibodies in the plasma of Brazilian brain tumor patients. Plasma samples were drawn from 24 untreated brain tumor patients and from 15 healthy donors without clinical signs of cancer. Western blotting techniques were used to detect p53 protein and anti-p53 antibodies. We found anti-p53 antibodies in 5/24 brain tumor patients. Age appears to affect the immune response, as four of six tumor patients under 16 years old had detectable anti-p53 antibodies, while these were found in only 1 of 18 adults (over 16 years old). We found no p53 protein in any of the serum samples from the brain tumors. Possibly the presence of this protein is affected by tumor type or by the organs that are sampled. Key words: Glioma, Anti-p53 antibodies, Western blotting INTRODUCTION Brain tumors are one of the leading causes of cancer death among children and adults. Gliomas are the most common type of primary brain tumor, accounting for over 40 to 50% of all central nervous neoplasms. There has been an increase in the incidence of brain tumors over the past 20 years. It is estimated that 36,200 new cases of brain tumors were diagnosed in 2001 in the United States (CBTRUS, 2000). Inactivation of the TP53 gene is the most common event in the development of several types of human cancer (Tilkin et al., 1995). The TP53 gene, located on the short arm of chromosome 17, was first identified in 1979, and is responsible for coding a 53-kDa nuclear phosphoprotein that acts as a negative regulator of the cell cycle, arresting the cell in G1 to allow DNA repair (Linzer and Levine, 1979). If repair fails, p53 may trigger cell death by apoptosis (Sidransky and Hollstein, 1996). Mutational inactivation of TP53 has been demonstrated in several types of brain tumors, including astrocytomas, oligodendrogliomas and medulloblastomas. In astrocytomas, mutations and loss of heterozygosity at 17p are genetic alterations detected early in the course of malignant progression. This affects gliomas, which carry these types of alterations in 25-40% of cases (Anker et al., 1993; Rasheed et al., 1999). Mutation at TP53 can lead to the production of abnormal protein, which has been shown to accumulate at higher levels in tumor cells when compared to the wild type p53 in normal cells, presumably due to increased protein stability (Angelopoulou and Diamands, 1997). p53 protein arising from the mutated gene is tumor specific, and can therefore be recognized as a foreign antigen, and under certain circumstances can become a target for a humoral immune response. Anti-p53 antibodies were first detected in the sera of breast cancer patients (Crawford et al., 1982). Since then, several studies have successfully detected anti-p53 antibodies in the plasma or sera of patients with various types of cancer. Davidoff et al. (1992) reported that the development of p53 antibodies is dependent upon complexing of the mutant protein with heat shock protein 70 (Hsp70), and is associated with this type of mutation. However, the complete mechanism of generation of anti-p53 antibodies has yet to be explained. We investigated circulating anti-p53 antibodies in the plasma of Brazilian brain tumor patients. Relationships between anti-p53 antibodies and clinical factors, such as tumor stage and age were also investigated. MATERIAL AND METHODS Patients and blood samples Twenty-four blood samples were drawn from untreated primary brain tumor patients (4 astrocytomas I, 6 astrocytomas II, 3 astrocytomas III, 7 glioblastomas, 3 ependymoma and 1 oligodendroglioma) and 15 were obtained from healthy donors, without clinical indications of cancer (negative control). All patients were registered and followed up at the Instituto Nacional de Cancer (INCa), Brazil. The blood (5 ml) was collected in tubes containing EDTA. Samples were separated by centrifugation and the plasma was removed, divided into aliquots and stored at -20°C, until analysis. Histopathological analysis Brain tumor diagnosis was made according to the World Health Organization Brain Tumor Classification (Kleihues et al., 1993). Production of recombinant p53 Recombinant human p53, expressed in Escherichia coli BL21 (DE3) after transformation with p53 cDNA in the vector pET 19b (gift from Dr. Robyn L. Ward, Saint Vicent’s Hospital, Australia), was used as a positive control. We used the protocol described in Molecular Cloning (Sambrook et al., 1989) to express the recombinant human p53 protein. The total extract from bacteria was lysed with a Tris-glycine-SDS buffer containing b-mercaptoethanol. The protein concentration was determined with the Bradford technique, using a protein standard curve generated with bovine serum albumin (BSA) (Bradford, 1976). The total extract was analyzed on a polyacrylamide gel, and its immunoreactivity was determined by immunoblotting with monoclonal antibody anti-p53 Pab 1801 (Santa Cruz Technology, Santa Cruz, CA, USA) (Laemmli, 1970; Towbin et al., 1979). Detection of p53 protein in plasma The plasma samples of all patients included in this study were diluted (1:20) with Tris-glycine-SDS buffer containing b-mercaptoethanol denatured by heating at 90°C for 5 min. Equal amounts of this mixture (10 ml) were loaded onto a one-dimensional 10% polyacrylamide electrophoresis gel, using the modified SDS buffer system of Laemmli (1970) at room temperature. As a positive control, 20 mg of total extract from bacteria that express recombinant human p53 protein was mixed with Tris-glycine-SDS buffer, as described above. The molecular weight of the proteins was determined by co-electrophoresis of prestained protein standards (Gibco BRL, Carlsbad, CA, USA). After electrophoresis, the proteins were transferred to a nitrocellulose membrane in a Mini-V 8-10 cube, according to manufacturer specifications (Towbin et al., 1979). The membrane was preincubated with 5% nonfat dried milk in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) to avoid nonspecific reactions and incubated for 1 h at 4°C with 1:100 monoclonal anti-p53 antibody (Santa Cruz Technology). After washing with TBST, the blot was incubated for 1 h with peroxidase-linked antimouse antibody (Amersham Pharmacya Biotech, UK). Protein detection was revealed with an enhanced chemiluminescence system (ECL, Amersham) and captured on X-ray Kodak film (X-Omat). Detection of anti-p53 antibodies in plasma To detect anti-p53 antibodies, 20 mg of the total extract from bacteria with the recombinant protein was subjected to SDS gel electrophoresis in a 10% polyacrylamide gel. Proteins were transferred from the gel to a nitrocellulose membrane by Western blotting, as described above. After protein transference, the individual protein lanes on the membrane were cut and separated into strips. These strips were incubated with a 1:100 solution of patient and control plasma and 5% nonfat dried milk in TBST for 1 h at room temperature. After washing with TBST, the strips were incubated with 1:1000 human anti-IgG antibody (BIOSYS/FRA) and developed, as described above. RESULTS The p53 protein was not detected by Western blot analysis in any of the patients with primary brain tumors or in the control plasma samples; however, anti-p53 antibodies were detected in 5 of 24 patients (Table 1). These antibodies were not found in the 15 control samples. We also analyzed the relationship between plasma antibodies against p53 and the tumor stage and degree of malignancy. Among the five patients that were found to have anti-p53 antibodies, three had astrocytomas II and III, which are the most aggressive tumor stages (Table 2).
The brain tumors were divided according to patient age. In pediatric tumors (under 16 years old) 4 of 6 patients had detectable anti-p53, while only 1 of 18 adults (above 16 years) had detectable antibodies. DISCUSSION Mutations of the p53 tumor suppressor gene, as well as overexpression of serum p53 antibodies and of p53 protein in tumor tissue, have been encountered in a variety of human malignancies (Soussi, 2000). Recent experiments have demonstrated p53 mutations in 35 to 40% of patients with primary brain tumors (Rasheed et al., 1999). Previous studies had revealed that the p53 gene is strongly associated with carcinogenesis and that patients have a poor prognosis when they have a p53 mutation in the primary tumor (Shibata et al., 1996). There is conflicting evidence concerning the presence of p53 protein in the peripheral blood of patients with cancer. This protein was not found in the serum of patients with lung and brain tumors (Levesque et al., 1996; Weller et al., 1998). However, low concentrations of p53 protein have been found in the serum of patients with breast cancer and hepatocellular cancer (Crawford et al., 1982; Volkman et al., 1993). Anti-p53 antibodies have rarely been detected in healthy donors or in patients without TP53 mutations. The molecular mechanisms that lead to p53 autoimmunity are still unknown. It has been proposed that the accumulation of p53 in tumor cells is an important factor in the development of an anti-p53 immune response. It is possible that accumulated p53 complexes with binding proteins, such as Hsp70, and thereby facilitates the induction of an immune response (Davidoff et al., 1992). We found no p53 protein in the plasma of 24 patients with brain tumors. However, anti-p53 antibodies were detected in 5 of these patients. This anti-p53 immune response was less frequent than has been found for other types of cancer (Rainov et al., 1995). Few patients with gliomas had an anti-p53 immune response. This response could be compromised by several factors, including release by gliomas and normal brain cells of immunosuppressive factors, such as transforming growth factor-b (TGF-b), lack of professional antigen presenting cells in the brain and exposure to steroid medication (Weller et al., 1998). Human glioblastoma cell lines have been found to secrete a potent immunosuppressive factor, which was found to be identical to TGF-b2 and which shares 71% homology with TGF-b1. The TGF-b family includes potent immunosuppressors that inhibit T and B cell activation, depress the activity of NK cells and decrease the production of interferon (Maxwell et al., 1992). Overexpression of TGF-b may allow tumor cells to escape from immune surveillance in the brain. There is little information about the stage of tumor development at which p53 antibodies appear in the plasma. We found that these antibodies appear more frequently in patients under 16 years old and in patients with advanced tumors (stages II and III). ACKNOWLEDGMENTS We thank Marcelo Soares Mota for technical assistance and Nathalie Henriques Silva for English review of this manuscript. Research supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Financiadora de Estudos e Projetos (FINEP). REFERENCES Angelopoulou, K. and Diamandis, E.P. (1997). Detection of the TP53 tumour suppressor gene product and p53 auto-antibodies in the ascites of women with ovarian cancer. Eur. J. Cancer 33: 115-121. Anker, L., Ohgaki, H., Ludeke, B., Hermann, H.-D., Kleihues, P. and Westhpal, M. (1993). p53 protein accumulation and gene mutations in human glioma cell line. Int. J. Cancer 55: 982-987. Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Bioch. 72: 248-254. CBTRUS (2000). Primary Brain Tumors in the United States Statistical Report. Central Brain Tumor Registry of the United States. Chicago, IL, USA. Crawford, L.V., Piw, D.C. and Bulbrook, R.D. (1982). Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer 30: 403-408. Davidoff, A.M., Iglehart, J.D. and Marks, J.R. (1992). Immune response to p53 is dependent upon p53/HSP70 complexes in breast cancers. Proc. Natl. Acad. Sci. USA 89: 3439-3442. Kleihues, P., Burger, P.C. and Scheithauer, B.W. (1993). Histological typing of tumors of the central nervous system. WHO International Histological Classification of Tumours. Springer-Verlag, Berlin-Heidelberg, Germany. Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. Levesque, M.A., D’Costa, M. and Diamandis, E.P. (1996). p53 protein is absent from the serum of patients with lung cancer. Br. J. Cancer 74: 1434-1440. Linzer, D.I.H. and Levine, A.J. (1979). Characterization of a 54K dalton cellular SV40 tumor antigen present in SV 40 cells and uninfected embryonal carcinoma cells. Cell 17: 43-52. Maxwell, M., Galanopulos, T., Neville-Golden, J. and Antoniades, H. (1992). Effect of the expression of transforming growth factor B-2 in primary human glioblastoma on immunosuppression and loss of immune surveillance. J. Neurosurg. 76: 799-804. Rainov, N.G., Dobberstein, K.U., Fittkan, M., Bahn, H., Holzhausen, H.J., Gantchev, L. and Burket, W. (1995). Absence of p53 autoantibodies in sera from glioma patients. Clin. Cancer Res. 1: 775-781. Rasheed, B.K., Wiltshire, R., Bigner, S. and Bigner, D. (1999). Molecular pathogenesis of malignant gliomas. Curr. Opin. Oncol. 11: 162-167. Sambrook, J., Fristsch, E.P. and Maniats, T. (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA. Shibata, Y., Kotanagi, H., Andoh, H., Koyama, K., Itoh, H. and Kudo, S. (1996). Detection of circulating anti-p53 antibodies in patients with colorectal carcinoma and the antibody’s relation to clinical factors. Dis. Colon Rectum 39: 1269-1274. Sidransky, D. and Hollstein, M. (1996). Clinical implications of p53 gene. Ann. Rev. Med. 47: 285-301. Soussi, T. (2000). P53 antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 60: 1777-1788. Tilkin, A., Lubin, R., Soussi, T., Lazar, V., Janin, N., Mathieu, M., Carlu, C., Roy, M., Kayibanda, M., Bellet, D., Guillet, J. and Paillerets, B. (1995). Primary proliferative T cell response to wild type p53 protein in patients with breast cancer. Eur. J. Immunol. 25: 1765-1769. Towbin, H., Staehelin, T. and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350- 4354. Volkman, M., Muller, M., Hoffmann, W.J., Meyer, M., Hagelstein, J., Rath, U., Kommerele, B., Zengtraf, H. and Gale, P.R. (1993). The humoral immune response to p53 in patients with hepatocellular carcinoma is specific for malignancy and independent of the alpha-fetoprotein status. Hepatology 18: 559-565. Weller, M., Borneman, A., Stander, M., Schabet, M., Dichgans, J. and Meyermann, R. (1998). Humoral immune response to p53 in malignant glioma. J. Neurol. 245: 169-172. |
|