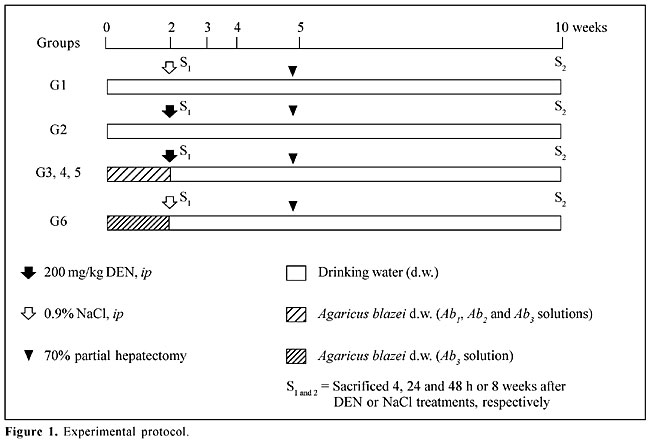
ABSTRACT. The effects of crude extracts of the mushroom Agaricus blazei Murrill (Agaricaceae) on both DNA damage and placental form glutathione S-transferase (GST-P)-positive liver foci induced by diethylnitrosamine (DEN) were investigated. Six groups of adult male Wistar rats were used. For two weeks, animals of groups 3 to 6 were treated with three aqueous solutions of A. blazei (mean dry weight of solids being 1.2, 5.6, 11.5 and 11.5 mg/ml, respectively). After this period, groups 2 to 5 were given a single ip injection 200 mg/kg DEN and groups 1 and 6 were treated with 0.9% NaCl. All animals were subjected to 70% partial hepatectomy at week five and sacrificed 4, 24 and 48 h or 8 weeks after DEN or 0.9% NaCl treatments (10th week after the beginning of the experiment). The alkaline comet assay and GST-P-positive liver foci development were used to evaluate the influence of the mushroom extracts on liver cell DNA damage and on the initiation of liver carcinogenesis, respectively. Previous treatment with the highest concentration of A. blazei (11.5 mg/ml) significantly reduced DNA damage, indicating a protective effect against DEN-induced liver cytotoxicity/genotoxicity. However, the same dose of mushroom extract significantly increased the number of GST-P-positive liver foci. Key words: Agaricus blazei, DNA damage, GST-P-positive liver foci, Hepatocarcinogenesis INTRODUCTION Among the mushroom species of higher Basidiomicetes, Agaricus blazei Murrill, a species native to Brazil, where it is popularly known as “sun mushroom”, has recently received attention in folk medicine due to its use in the treatment of ailments. Since 1965, strains have been exported from Brazil to Japan, where this mushroom has become popularly known as “Himematsutake” or “Agarikusutake”. This edible mushroom is often consumed as food and tea in different parts of the world, especially because of its reported medicinal properties. In Brazil, infusion of the dried fruiting bodies of the mushroom A. blazei has been popularly consumed both as a stimulant and for auxiliary treatment of various diseases, including cancer. Nevertheless, no epidemiological and few in vivo experimental data exist on the beneficial effects of the crude aqueous extract of this mushroom. Recently, we demonstrated that this extract provides significant protection against mutagenicity induced by cyclophosphamide and methyl methanesulfonate, both in vivo and in vitro (Delmanto et al., 2001; Menoli et al., 2001; Martins de Oliveira et al., 2002). Various polysaccharides and protein-bound polysaccharides isolated from mycelia and fruiting bodies of A. blazei have shown anti-tumor activity in tumor-bearing mice by host immune response activation (Kawagishi et al., 1989; Itoh et al., 1994, 1997; Fujimiya et al., 1998; Mizuno et al., 1998). Dielthylnitrosamine (DEN) is a potent genotoxic carcinogen that has been used as initiating agent in some two-stage (initiation-promotion) alternative protocols for hepatocarcinogenesis (Ito et al., 1988; Dragan et al., 1991). It has been reported that after its metabolic biotransformation, DEN produces the promutagenic adducts O6-ethyldeoxyguanosine and O4- and O6-ethyldeoxythymidine that may initiate liver carcinogenesis (Dragan et al., 1994; Verna et al., 1996). Consequently, the analysis of DNA damage may be relevant to evaluate the modifying influences of chemopreventive agents on the initiation stage of cancer (Moore et al., 1999). The comet assay or single cell gel electrophoresis assay is a rapid and sensitive procedure for quantifying DNA lesions in individualized cells, both in vitro and in vivo (Tice et al., 1991; Fairbairn et al., 1995; Gontijo et al., 2001). The alkaline comet assay version was specially developed for detection of the DNA single-strand breaks and alkali-labile sites (Singh et al., 1988) and is also indicated to evaluate in vivo genotoxicity induced by carcinogen exposure (Anderson et al., 1998; Tsuda et al., 2000). The DEN-partial hepatectomy (PH) model has proven to be a consistent bioassay for the detection of chemical hepatocarcinogens and for the assessment of the beneficial potential of chemopreventive agents (Ito et al., 1988, 1996; Moore et al., 1999). This 8-week-long medium-term rat liver assay uses as endpoint marker the development of putative preneoplastic foci of altered hepatocytes that express the placental form of the enzyme glutathione S-transferase (GST-P) (Ito et al., 1988). It has been indicated as a practical approach for the assessment of the potential hazard or benefit of chemicals, when associated with other surrogate end-points (Moore et al., 1999). The standard DEN-PH assay protocol uses 200 mg/kg DEN to establish the initiation of the hepatocarcinogenesis process (Ito et al., 1988). In order to determine the protective influence of the aqueous solutions of the mushroom A. blazei against liver damage and preneoplasia development induced by the DEN dose level used in the DEN-PH assay, we evaluated DNA damage and foci of altered hepatocyte development by the comet assay and by the immunohistochemical expression of the placental enzyme, glutathione S-transferase, respectively. MATERIAL AND METHODS Animals and treatment Male 4-week-old Wistar rats were obtained from CEMIB (UNICAMP, Campinas, SP, Brazil). The animals were kept in polypropylene cages (five animals/cage) covered with metallic grids in a room maintained at 22 ± 2oC, 55 ± 10% humidity, and with a 12-h light-dark cycle. They were fed with commercial Purina chow (Labina, Paulínia, SP, Brazil) and water ad libitum during a 2-week acclimation period. The animals were randomly allocated into six groups (Figure 1). For two weeks, animals of groups 3 to 6 were treated with aqueous solutions of the A. blazei, with a mean dry weight of water-extractable solids of 1.2, 5.6, 11.5 and 11.5 mg/ml, respectively. After this period, groups 2 to 5 were given a single ip injection of 200 mg/kg DEN (Sigma, St. Louis, MO, USA), and groups 1 and 6 were treated with 0.9% NaCl only (DEN vehicle). All animals were subjected to 70% partial hepatectomy at week five and sacrificed 4, 24 and 48 h or 8 weeks after DEN or 0.9% NaCl treatments (10th week after the beginning of the experiment). The University Ethics Committee for Animal Research approved the protocols used in this study (Protocol No. 99/22).
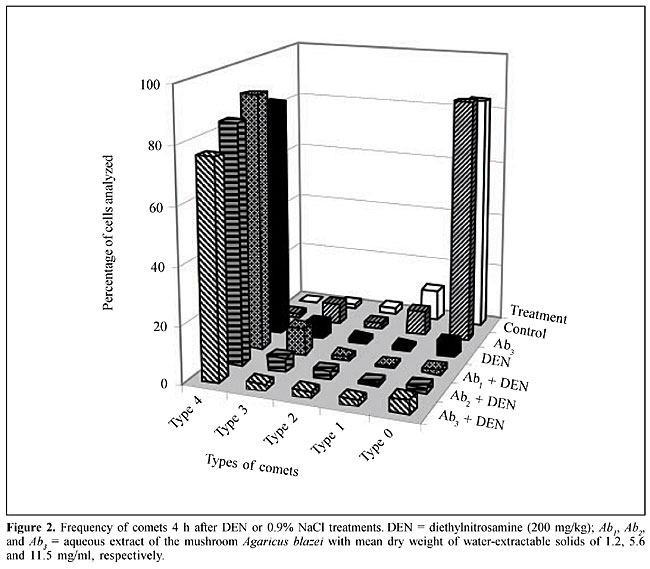
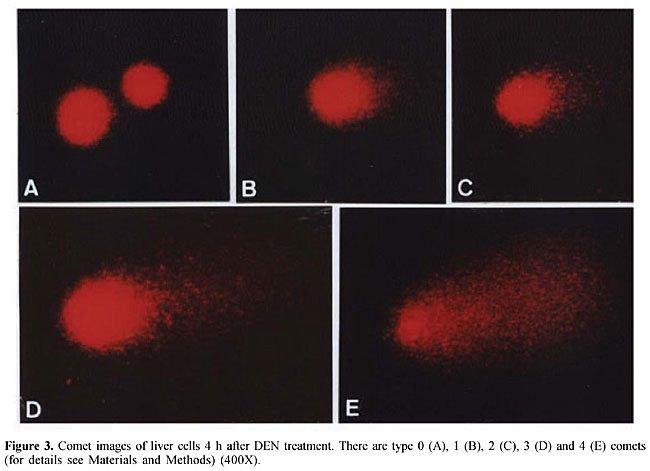
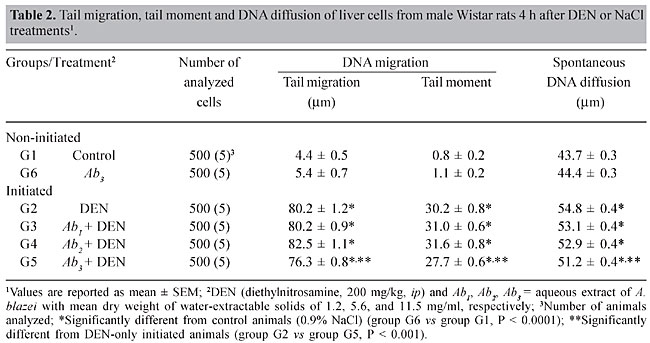
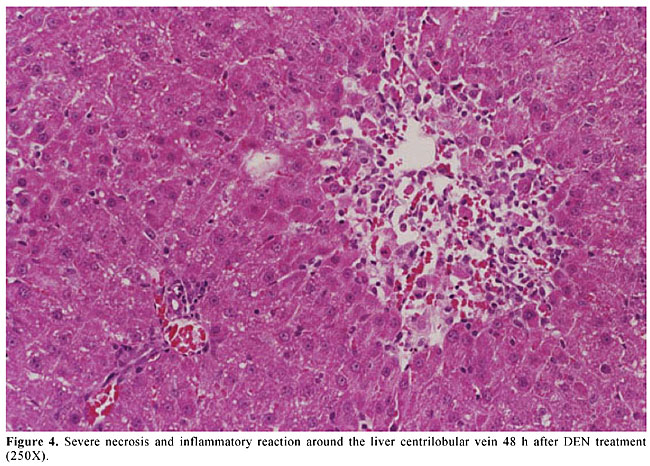
A sample of A. blazei Murrill (lineage 99/26) was obtained from the Departamento de Produção Vegetal, Faculdade de Ciências Agronômicas, UNESP, Botucatu, SP, Brazil. Twenty-five grams of powdered dry fruiting bodies of A. blazei mushroom was added to 1000 ml of deionized water (2.5% w/w) and left for 2 h at room temperature. This preparation corresponds to the popular form of use of A. blazei for beneficial health effects. This solution, referred from here on as “the crude aqueous extract”, was then centrifuged (800 g for 10 min) and filtered (commercial non-sterile filter). The final solution was provided as 10% (Ab1), 50% (Ab2) and 100% (Ab3) of the full 2.5 (w/w) aqueous extract of A. blazei. The mean amount of water-extractable solids in Ab1, Ab2, and Ab3 solutions was 1.2, 5.6, and 11.5 mg/ml, respectively. The yields were 4.8, 22.4 and 46%, respectively. The solutions were prepared daily and offered to rats ad libitum, in aluminum foil-wrapped bottles to avoid light decomposition. They were the sole source of drinking fluid, starting two weeks before DEN initiation. Comet assay Four hours after DEN or NaCl treatments, five animals of each experimental group were sacrificed and a small piece of the left hepatic lobe was collected and placed onto a small Petri disc with ice-cold mincing solution (Ca2+- and Mg2+-free HBSS containing 20 mM EDTA and 10% DMSO). The liver samples were cut into smaller pieces, using a disposable microtome razor blade, and the solution was aspirated. Then, a fresh mincing solution was added and the liver samples were minced again to finer pieces. Resulting cell suspensions were collected and filtered (100 µm nylon mesh). All samples were stocked on ice in appropriate conditions to avoid light until the comet assay procedures. The quantity of liver cells in the cell suspensions was determined in Giemsa-stained smears. The viability of the liver cells was indirectly determined by analyzing the comet images after electrophoresis (Vaghef and Hellman, 1998) and by spontaneous DNA diffusion without DNA denaturation and electrophoresis (Vasques and Tice, 1997). The comet image was considered to be from a nonviable cell when it presented a “cloudy” appearance or a very small head and a tail like a balloon (necrotic or apoptotic cells). The viability of the cell suspension was considered acceptable when the frequency of such images was less than 2% (Vaghef and Hellman, 1998). Higher values were considered as indicative of cytotoxicity due to the carcinogen treatment. The extent of spontaneous DNA diffusion was evaluated by measuring the diameter of the liver cell “nucleoids”, considering that cells containing extremely low molecular weight DNA associated with apoptosis or necrosis develop spontaneous DNA diffusion into the agarose gel and consequently have larger diameters (Vasques and Tice, 1997). The comet assay was performed under alkaline conditions according to a previously described standard protocol (Speit and Hartmann, 1999). Briefly, an aliquot of 5 µl of each prepared hepatic cell suspension was mixed with 120 µl of 0.5% low melting point agarose at 37oC and layered onto conventional microscope slides, precoated with 1.5% normal melting point agarose. The slides were placed overnight in freshly prepared cold lysing solution (1% Triton X-100, 2.5 mM NaCl, 0.1 mM Na2EDTA, 10 mM Tris with 10% DMSO, pH 10.0) and then in a horizontal electrophoresis cube with alkaline electrophoresis solution (0.3 M NaOH, 1 mM Na2EDTA, pH >13) at 4oC for 20 min. The electrophoresis was performed at 25 V and 300 mA for 20 min. After electrophoresis, the slides were washed twice for 5 min in neutralizing buffer (0.4 M Tris-HCl, pH 7.5), fixed for 5 min in absolute alcohol, air-dried, and stored at room temperature. In order to evaluate extremely low molecular weight DNA diffusion, two slides from each animal were removed after lysis procedure, rinsed with neutralizing solution, fixed and air-dried, and stored until analysis. Immediately before analysis, the DNA was stained with 50 µl of 20 µg/ml ethidium bromide. The slides were examined with a 40X objective lens with epi-illuminated fluorescence microscopy (Olympus-Bx60, excitation filter: 515-560 nm; barrier filter: 590 nm) attached to a color CCD video camera and connected to an image analysis system (Comet II, Perspective Instruments, UK). Coded slides were scored blindly and 100 hepatic cell images were randomly analyzed for each animal (50 images per slide). The comets were analyzed by a visual scoring method and computerized image analysis. The comets were classified into five categories, defined as types 0, 1, 2, 3 and 4 - where 0 indicates no or very low damage, 1, 2 and 3 indicate low, medium and long DNA migration, respectively, and 4 indicates apoptotic or necrotic DNA migration (Speit and Hartmann, 1999). The metrics for comet analysis included spontaneous DNA migration (measure of diameters larger than 34 microns of the “nucleoids”, expressed in microns), tail migration (distance from the end of the head to the end of the tail, expressed in microns), and tail moment (product of DNA density in the tail and the mean distance of DNA migration in the tail, expressed in arbitrary units). Histology and GST-P-positive foci analysis After sacrifice at the end of the 10th week, samples of each of the liver lobes were weighed and fixed in 10% phosphate-buffered formalin for hematoxylin-eosin (HE) staining and immunohistochemical demonstration of GST-P-positive foci, using the avidin-biotin complex method (Hsu et al., 1981). Paraffin-embedded liver samples were cut into 5-µm thick sections, placed on poly/D/lysine-coated slides, deparaffinized in xylene and rehydrated with graded alcohol to water. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in phosphate-buffered saline (PBS) for 5 min. Nonspecific protein binding was minimized by the use of 1% nonfat dried milk in PBS for 60 min at 4oC. Slides were incubated with rabbit anti-rat GST-P primary antibody (Medical and Biological Laboratories Co., Tokyo, Japan) diluted at 1:1000 in 1% bovine serum albumin (BSA; Sigma) overnight at 4oC. Then, the slides were successively incubated with a biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories Inc., CA, USA) diluted at 1:200 in 1% BSA for 60 min, followed by an avidin-biotin-horseradish peroxidase complex kit (Vector Laboratories Inc.) diluted at 1:100 in PBS for 45 min. Subsequent chromagen color development was made using 0.038% 3-3’-diaminobenzidine tetrahydrochloride (Sigma) and 0.025% hydrogen peroxide in 0.1 M Tris-HCl, pH 7.4, for 4 min. The sections were counterstained with Harris’s hematoxylin, dehydrated, cleared in xylene, and a cover slip fixed with a xylene-based mounting medium. GST-P-positive foci larger than 0.15 mm2 in diameter were measured using a Nikon photomicroscope (Microphot-FXA) connected to a KS-300 apparatus (Kontron Elektronic, Germany). Data are reported as number and area (mm2) of preneoplastic GST-P-positive foci per liver section (cm2). Statistical analysis Comparisons of body and liver weights among groups were carried out using analysis of variance and Student t-test. The DNA migration data and the number and area of the GST-P-positive foci were analyzed by Mann-Whitney and Kruskal-Wallis tests (Zar, 1984). The contrast between groups was analyzed by the Tukey test. A significant difference between the groups was assumed when P < 0.05. RESULTS Food and liquid consumption and body and liver weights The two-week A. blazei treatment had no influence on the mean body weight gain (Table 1), on food consumption (~25 g/rat/day for all the experimental groups) or on liver morphology, evaluated by HE (data not shown). Significantly increased ingestion of the aqueous extract of A. blazei was observed for 50 and 100% of the full aqueous extract (2.5 w/w) of A. blazei (Ab2 and Ab3 solutions) (group G1 vs group G6, P < 0.001; group G2 vs groups G4 and G5, P < 0.001) (Table 1). The average ingestion of A. blazei (i.e., water-extractable material) calculated from the mean ingestion of the aqueous solutions of A. blazei was approximately 0.18, 0.90 and 1.97 g of solids/kg/day (Table 1). 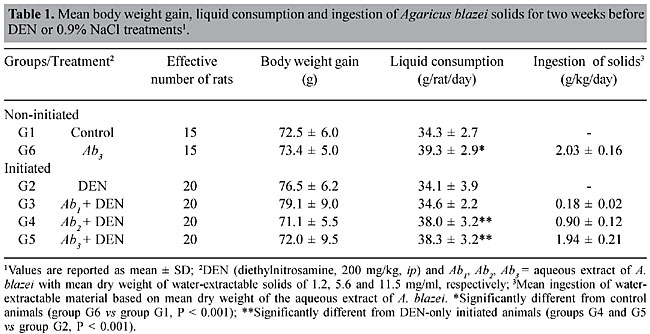 No differences were found in the mean body and liver weights between non-initiated (groups G1 and G2) and DEN-initiated animals (group G2 to group G5) at the end of the experimental period (data not shown). DNA damage evaluation In untreated animals (group G1), there was a low frequency of necrotic hepatocytes, indicated by type 4 comet images (Figure 2). DEN treatment induced a higher frequency of type 4 comet images (necrosis/apoptosis process) and increased DNA migration in the alkaline comet assay, when compared to the non-initiated animals (group G1 vs group G2) (Figures 2 and 3). Necrosis/apoptosis of liver cells in DEN-treated animals was also indicated by increased spontaneous DNA diffusion (Table 2) and by extensive centrilobular liver necrosis observed 48 h after DEN treatment (Figure 4).
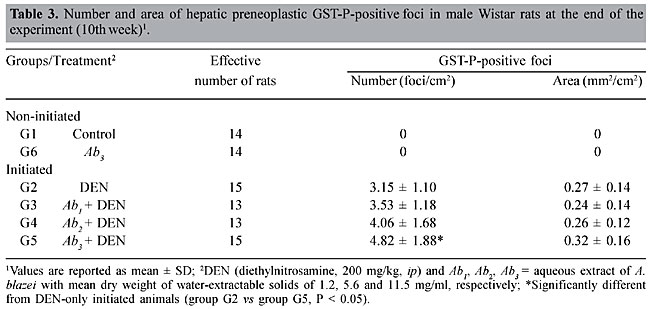
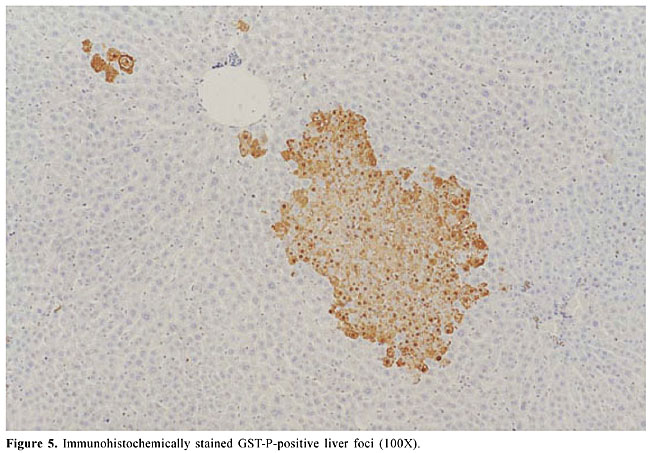
Agaricus blazei treatment alone did not change the basal level of DNA damage in liver cells, evaluated by DNA migration in the comet assay (Table 2 and Figure 2). Significantly smaller values of the DNA migration parameters (tail migration and tail moment) and of spontaneous DNA diffusion analysis without electrophoresis were observed in rats that received the highest concentration of the mushroom A. blazei (Ab3 solution) before 200 mg/kg DEN, than in rats solely treated with this dose of carcinogen (group G2 vs group G5, P < 0.001, Table 2). GST-P quantitative data Non-DEN-treated control and A. blazei-treated animals (groups G1 and G6) did not develop preneoplastic GST-P-positive liver foci (Table 3, Figure 5). Higher values of the number of but not the area of GST-P-positive liver foci per cm2 of liver sections were observed in rats that received the highest concentration of A. blazei (Ab3 solution) previously to 200 mg/kg DEN than in rats solely treated with this dose of carcinogen (group G2 vs group G5, P < 0.001, Table 3).
DISCUSSION The potential of aqueous extracts of the mushroom A. blazei to ameliorate DNA damage and GST-P foci development induced by DEN was investigated in male Wistar rats. Natural products have been traditionally accepted as remedies due to the popular belief that they produce few adverse side effects. Therefore, understanding the potential beneficial or adverse influence of natural products extensively used by human population is very important to implement public health safety measures. We did not observe adverse effects of treatment with relatively high doses of a crude aqueous solution of the mushroom A. blazei, determined from body weight, liver morphology, DNA damage and GST-P foci data. In the comet assay, cells with damaged DNA displayed increased migration of DNA fragments (comet tail) from the nucleus (comet head), which may also be a feature of DNA fragmentation associated with the necrotic/apoptotic death process (Olive et al., 1993; Fairbairn et al., 1996). In contrast with the conventional dye exclusion assay to evaluate cell viability (e.g., Trypan blue), we used two other approaches to evaluate concurrent liver cytotoxicity due to the DEN treatment. These alternative cytotoxicity assays included liver histological analysis for the detection of necrosis/apoptosis and the single-cell neutral diffusion assay to detect cells with low molecular weight DNA fragments, indicative of apoptosis or necrosis (Tice et al., 2000). Treatment with 200 mg/kg DEN induced liver cytotoxicity, evidenced by increased spontaneous DNA diffusion and also by severe centrilobular liver necrosis. The increased DNA migration observed 4 h after DEN treatment was likely a sum of genotoxicity and cytotoxicity induced by the carcinogen (Hartmann and Speit, 1997). A protective effect of the treatment with the higher A. blazei aqueous extract concentrations against DEN cytotoxicity/genotoxicity can be postulated because there was a diminished tail extension and diminished DNA content in the tails of the hepatocyte comets in A. blazei-treated animals when compared to the animals treated only with the standard 200-mg/kg DEN dose. It was previously found that A. blazei extracts inhibit the mutagenicity of benzo(a)pyrene in the Ames Salmonella microsome assay (Osaki et al., 1994) as well as mutagenicity induced by cyclophosphamide in the mouse bone marrow micronucleus test (Delmanto et al., 2001). In general, crude extracts and polysaccharides isolated from mushrooms have protective activity against chemical liver injury (Yeung et al., 1995; Ooi, 1996). Treatment with crude extracts of Lentinus edodes, Grifola frondosa, Tricholoma lobayence and an isolated polysaccharide peptide of Coriolus versicolor provides significant protection against paracetamol-induced hepatotoxicity by preventing a decrease in hepatic reduced glutathione and by increasing the conjugation and excretion of the drug reactive metabolites (Yeung et al., 1995; Ooi, 1996). The protective effect of A. blazei against the cytotoxicity induced by DEN could result from a modification in DEN metabolism due to active principles in the A. blazei extracts. Altered foci of hepatocytes are believed to be early markers of the rodent liver cancer development (Bannasch and Zerban, 1992). A variety of phenotypic abnormalities identify the altered foci of hepatocytes, including abnormal expression of enzymes such as GST-P (Sato, 1988). In the DEN-PH model, the necrogenic dose of 200 mg/kg DEN was adopted because it induces a large number of GST-P-positive foci, favoring the analysis of the anti-cancer promoting potential of chemicals (Hasegawa and Ito, 1992). In a parallel study on DEN-induced cancer initiation of Wistar rats, we found that a previous treatment with a moderate dose of A. blazei (5.6 mg/ml) can exert hepatoprotection against liver toxicity and against the development of single GST-P-positive hepatocytes induced by 100 mg/kg DEN (Barbisan et al., 2002). Therefore, it was assumed that higher doses of A. blazei would also exert a beneficial influence against the initiation step of liver carcinogenesis by the standard protocol for medium-term hepatocarcinogenesis assay, which uses 200 mg/kg DEN for initiation. However, an increased number of the GST-P-positive foci were observed in animals treated with the higher doses of A. blazei before DEN. This could be attributed to the toxic environment induced by the higher dose of the carcinogen, when compared to the previous study. Also, the protection against liver cytotoxicity by the crude mushroom extract in the animals treated with the necrogenic dose of DEN could increase the survival of initiated cells (putative preneoplastic cells) through the process of liver initiation, resulting in a significant enhancement of GST-P-positive foci. Numerous efforts towards reducing the chemical initiation of the carcinogenesis process have been unfruitful (Schmitt et al., 1993; Tessitore et al., 1996, 1997; Hambly et al., 1997). Schmitt et al. (1993) and Tessitore et al. (1996, 1997) were unable to detect an influence of fasting on the initiation stage of rat liver and mammary carcinogenesis when relatively high doses of carcinogens were used. Hambly et al. (1997) showed that diets containing low risk factors for colorectal cancer (e.g., low in fat and high in fiber and calcium) reduced dimethylhydrazine-induced DNA damage, as assessed by the comet assay, but did not change the aberrant crypt multiplicity, a preneoplastic marker for colorectal cancer in carcinogen-induced rodent models. Based on these results it appears that DNA damage does not always correlate with the development of histologically detected preneoplasia. We also showed that DNA damage does not correlate with GST-P-positive foci development. It is possible that chemically induced models using a relatively high dose of carcinogen are not well suited to study factors relevant to the initiation events (Tessitore et al., 1996, 1997; Rijken et al., 1999). Apparently the protective influence of aqueous extracts of A. blazei against DEN genotoxicity, cytotoxicity and carcinogenicity is due to different mechanisms and is dependent on both the dose of the chemopreventive agent and of the carcinogen used. The highest concentration of A. blazei extract (11.5 mg/ml) demonstrated pro-carcinogenic properties by reducing the elimination of damaged cells (there was less apoptosis/necrosis of liver cells after DEN injection in rats receiving the mushroom extract) leading to the formation of an increased number of preneoplastic lesions. ACKNOWLEDGMENTS We gratefully acknowledge the technical support provided by Paulo Roberto Cardoso and Mara Luíza Falagueira Ardanaz. Research supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant No. 98/07726-5) and Fundação para o Desenvolvimento da UNESP (FUNDUNESP, grant No. 119/99-DFP). C. Scolastici (PIBIC/UNESP), D.M.F Salvadori, L.R. Ribeiro and J.L.V. de Camargo were recipients of fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). REFERENCES Anderson, D., Tian-Wei, Y. and McGregor, D.B. (1998). Comet assay responses as indicators of carcinogen exposure. Mutagenesis 13: 539-555. Bannasch, P. and Zerban, H. (1992). Predictive value of hepatic preneoplastic lesions as indicators of carcinogenic response. In: Mechanism of Carcinogenesis in Risk Indetification (Vainio, H., Magee, P.N., McGregor, D.B. and McMichal, A.J., eds.), International Agency for Research on Cancer (IARC) Sciences Publications, Lyon, France, pp. 389-427. Barbisan, L.F., Miyamoto, M., Scolastici, C., Salvadori, D.M.F., Ribeiro, L.R., da Eira, A.F. and de Camargo, J.L.V. (2002). Influence of aqueous extract of Agaricus blazei on rat liver toxicity induced by different doses of diethylnitrosamine. J. Ethnopharmacol. 83: 25-32. Delmanto, R.D., de Lima, P.L.A., Sugui, M.M., da Eira, A.F., Salvadori, D.M.F., Speit, G. and Ribeiro, L.R. (2001). Antimutagenic effects of Agaricus blazei Murill mushroom on the genotoxicity induced by cyclophosphamide. Mutat. Res. 496: 15-21. Dragan, Y.P., Rizui, T. and Xu, Y.H. (1991). An initiation-promotion assay in rat live as a potential complement to the 2-year carcinogenesis bioassay. Fundam. Appl. Toxicol. 16: 525-547. Dragan, Y.D., Campbell, H.A., Baker, K., Vaughan, J., Mass, M. and Pitot, H.C. (1994). Focal and non-focal hepatic expression of placental glutathione S-transferase in carcinogen-treated rats. Carcinogenesis 15: 2587-2591. Fairbairn, D.W., Olive, P.L. and O’Neill, K.L. (1995). The comet assay: a comprehensive review. Mutat. Res. 339: 37-59. Fairbairn, D.W., Walburger, D.K., Fairbairn., J.J. and O’Neill, K.L. (1996). Key morphologic changes and DNA strand breaks in human lymphoid cells: discriminating apoptosis from necrosis. Scanning 18: 407-416. Fujimiya, Y., Suzuki, Y., Oshiman, K., Kobori, H., Moriguchi, K., Nakashima, H., Matumoto, Y., Takahara, S., Ebina, T. and Katakura, R. (1998). Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol. Immunother. 46: 147-159. Gontijo, A.M.M.C., Elias, F.N., Salvadori, D.M.F., de Oliveira, M.L.C.S., Correa, L.A., Goldberg, J., Trindade, J.C.S. and de Camargo, J.L.V. (2001). Single-cell gel (comet) assay detects primary DNA damage in nonneoplastic urothelial cells of smokers and ex-smokers. Cancer Epidemiol. Biomakers Prev. 10: 987-993. Hambly, R.J., Rumney, C.J., Cunninghame, M., Fletcher, J.M.E., Rijken, P.J. and Rowland, I.R. (1997). Influence of diets containing high and low risk factors for colon cancer on early stages of carcinogenesis in human flora-associated (HFA) rats. Carcinogenesis 18: 1535-1539. Hartmann, A. and Speit, G. (1997). The contribution of cytotoxicity to DNA - effects in the single cell gel test (comet assay). Toxicol. Lett. 90: 183-188. Hasegawa, R. and Ito, N. (1992). Liver medium-term bioassay in rats for screening of carcinogens and modifying factors in hepatocarcinogenesis. Food Chem. Toxicol. 30: 972-992. Hsu, S.M., Raine, L. and Fanger, N. (1981). Use of avidin-biotin-peroxidase complex (ABC) and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 29: 557-580. Ito, H., Shimura, K., Itoh, H. and Kawade, M. (1997). Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade Strain 101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Res. 17: 277-284. Ito, N., Tsuda, H., Tatematsu, M., Inoue, T., Tagawa, Y., Aoki, T., Uwagawa, S., Kagawa, M., Ogiso, T., Masui, T., Imaida, K. and Asamoto, M. (1988). Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats: an approach for a new medium-term bioassay system. Carcinogenesis 9: 387-394. Ito, N., Hasegawa, R., Imaida, K., Hirose, M. and Shirai, T. (1996). Medium-term liver and multi-organ carcinogenesis bioassay for carcinogens and chemopreventive agents. Exp. Toxicol. Pathol. 48: 113-119. Itoh, H., Ito, H., Amano, H. and Noda, H. (1994). Inhibitory action of a (1®6)-b-D-glucan-protein complex (FIII-2-b) isolated from Agaricus blazei Murill (“Himematsutake”) on Meth A fibrosarcoma-bearing mice and its antitumor mechanism. Jpn. J. Pharmacol. 66: 265-271. Kawagishi, H., Inagaki, R., Kanao, T., Mizuno, T., Shimura, K., Ito, H., Hagiwara, T. and Nakamura, T. (1989). Fractionation and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydr. Res. 186: 267-273. Martins de Oliveira, J., Jordão, B.Q., Ribeiro, L.R., Ferreira da Eira, A. and Mantovani, M.S. (2002). Anti-genotoxic effects of aqueous extracts of sun mushroom (Agaricus blazei Murill lineage 99/26) in mammalian cells in vitro. Food Chem. Toxicol. 40: 1775-1780. Menoli, R.C., Mantovani, M.S., Ribeiro, R.L., Speit, G. and Jordão, B.Q. (2001). Antimutagenic effects of the mushroom Agaricus blazei Murrill extracts on V79 cells. Mutat. Res. 496: 5-13. Mizuno, M., Morimoto, M., Minato, K. and Tsuchida, H. (1998). Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci. Biotechnol. Biochem. 63: 434-437. Moore, M.A., Tsuda, H., Tamano, S., Hagiwara, A., Imaida, K., Shirai, T. and Ito, N. (1999). Marriage of a medium-term liver model to surrogate markers - a practical approach for risks and benefit assessment. Toxicol. Pathol. 27: 237-242. Olive, P.L., Frazer, G. and Banath, J.P. (1993). Radiation-induced apoptosis mesured in TK6 human B lymphoblast cells using the comet assay. Radiat. Res. 136: 130-136. Ooi, V.E.C. (1996). Hepatoprotective effect of some edible mushrooms. Phytother. Res. 10: 536-538. Osaki, Y., Kato, T., Yamamoto, K., Okubo, J. and Miyazaki, T. (1994). Antimutagenic and bactericidal substances in the fruit body of a basidiomycete Agaricus blazei. Yakugaku-Zasshi 114: 342-350. Rijken, P.J., Timmer, W.G., van de Kooij, A.J., van Benschop, I.M., Wiseman, S.A., Mijers, M. and Tijburg, L.B.M. (1999). Effect of vegetable and catenoid consumption on aberrant crypt multiplicity, a surrogate end-point marker for colorectal cancer in azoxymethane-induced rats. Carcinogenesis 20: 2267-2272. Sato, K. (1988). Glutathione S-transferase and hepatocarcinogenesis. Jpn. J. Cancer Res. 79: 556-572. Schmitt, L.F.C., Estevão, D., Kobayasi, S., Curi, P. and de Camargo, J.L.V. (1993). Altered foci of hepatocytes in rats initiated with diethylnitrosamine after prolonged fasting. Food Chem. Toxicol. 31: 629-636. Singh, N.P., Mccoy, M.T., Tice, R.R. and Schneider, E.L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175: 184-191. Speit, G. and Hartmann, A. (1999). The comet assay (single-cell gel test). A sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol. 113: 203-212. Tessitore, L., Tomasi, C., Greco, M., Sesca, E., Laconi, E., Maccioni, O., Ramo, R. and Pani, P. (1996). A subnecrogenic dose of diethylnitrosamine is able to initiate hepatocarcinogenesis in rat when coupled with fasting/refeeding. Carcinogenesis 17: 289-292. Tessitore, L., Chiara, M., Sesca, E., Premoselli, F., Binasco, V. and Dianzani, M.U. (1997). Fasting during promotion, but not during initiation, enhances the growth of methylnitrosourea-induced mammary tumours. Carcinogenesis 18: 1679-1681. Tice, R.R., Andrews, P.W., Hirai, O. and Singh, N.P. (1991). The single cell gel (SCG) assay: an electrophoretic technique for the detection of DNA damage in individual cells. Adv. Exp. Med. Biol. 283: 157-164. Tice, R.R., Argurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Miymae, Y., Rojas, E., Ryu, J.C. and Sasaki, Y.F. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35: 206-221. Tsuda, S., Matsusaka, N., Madarame, H., Miyamae, Y., Ishida, K., Satoh, M., Sekihashi, K. and Sasaki, Y.F. (2000). The alkaline single cell electrophoresis assay with eight mouse organs: results with 22 mono-function alkylating agents (including 9 diakyl-nitrosoamines) and 10 DNA crosslinkers. Mutat. Res. 467: 83-98. Vaghef, H. and Hellman, B. (1998). Detection of styrene and styrene oxide-induced DNA damage in various organs of mice using the comet assay. Pharmacol. Toxicol. 83: 69-74. Vasques, M. and Tice, R.R. (1997). Comparative analysis of apoptosis versus necrosis using the single cell gel (SCG). Environ. Mol. Mutagen. 29: 28 (Abstract). Verna, L., Whysner, J. and Williams, G.M. (1996). N-Nitrodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 71: 57-81. Yeung, J.H.K., Chiu, L.C.M. and Ooi, V.E.C. (1995). Effects of polysaccharide peptide (PSP) on in vivo sulphation and glucuronidation of paracetamol in the rat. Eur. J. Drug Metab. Pharmacokinet. 20: 287-292. Zar, J.H. (1984). Biostatististal Analysis. 2nd edn. Pretince Hall, Englewood Cliffs, NJ, USA. |
|