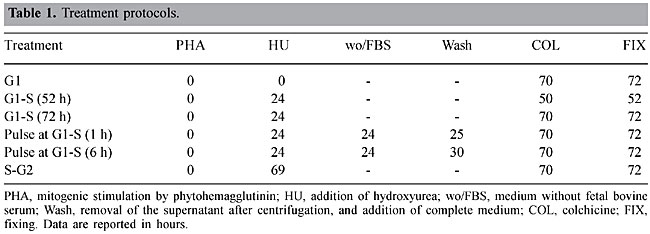
ABSTRACT. Hydroxyurea is considered an antineoplastic drug, which also plays an important role in the treatment of sickle cell anemia patients. We evaluated and compared the clastogenic and cytotoxic effects of hydroxyurea, using chromosomal aberrations and mitotic index, respectively, as endpoints. In vitro short-term cultures of lymphocytes were exposed to several concentrations of this drug, at various cell cycle phases. There was a significant increase in the cytotoxicity of hydroxyurea at G1 and G1/S as well in the G2 phase of the cell cycle. Hydroxyurea did not significantly increase chromosome aberrations. There was an S-dependent cytotoxic effect of hydroxyurea, which is expected based on the known activity of hydroxyurea as an inhibitor of ribonucleotide reductase. Key words: Hydroxyurea, Mitotic index, Chromosome aberrations, Sickle cell anemia INTRODUCTION Hydroxyurea (HU) is a drug with biological and anti-neoplastic potential. This chemical compound was discovered in the middle of the last century and it has been used in the treatment of a wide variety of neoplastic and hematological diseases (Donehower, 1992; Kennedy, 1992). Currently, HU is used to treat sickle cell anemia patients to induce the production of fetal hemoglobin (HbF), considerably improving their clinical symptoms (Yarbro, 1992). The specific action of HU is on ribonucleotide reductase. Ribonucleotides are reduced by ribonucleotide reductase to deoxyribonucleotides; this reduction reaction is impeded by HU, which limits DNA biosynthesis. Consequently HU is an S-phase-specific cytotoxic and antineoplastic agent that interrupts the cell cycle at the G1 and S phases (Yarbro, 1992). Although HU is highly beneficial for the combat of diseases that are among the principal causes of death throughout the world, it can also affect the DNA molecule (genotoxic action) causing irreversible alterations in the genetic material, which could have serious consequences for the organisms in which these alterations occur (Donehower, 1992). Despite its recognized effect on genetic material, few studies on the genotoxicity of HU have been carried out. Therefore, the aim of the present study was to evaluate and compare the genotoxic potential of HU on in vitro short-term cultures of lymphocytes using cytogenetic endpoints. MATERIAL AND METHODS Samples Lymphocytes from peripheral blood were obtained from a 19-year-old male and a 24-year-old female; they were cultivated in complete medium containing 76.8% HAM-F10 medium, supplemented with 0.01 mg/ml streptomycin, 0.005 mg/ml penicillin, 19.2% fetal bovine serum, and 4% phytohemagglutinin. Cytological preparations were prepared according to Moorhead et al. (1960). Chemical agent The antitumoral drug Hydrea® (HU) was obtained in gelatine capsules containing 500 mg of fine crystals; all of the solutions were prepared with sterile, double-distilled water. Treatments and biological tests The cells were treated with HU at doses of 6.25, 12.5, 25, and 50 µg/ml in HAM-F10 medium, at interphase phases G1, S and G2. These concentrations were determined based on experiences with mutagenic tests for antitumoral substances (Antunes and Takahashi, 1998). Untreated cultures were set up as negative controls. Among the phases of the cell cycle, G2 is the most adequate for detecting the mutagenic effects of this drug, as this drug is S-dependent and it generally inhibits progression of the cell cycle when added to the cell culture at phases prior to G2 (Lindenhahn and Schubert, 1983). Therefore, at this phase, the cells were also treated with drug doses of 100, 150 and 200 µg/ml culture medium (Table 1).
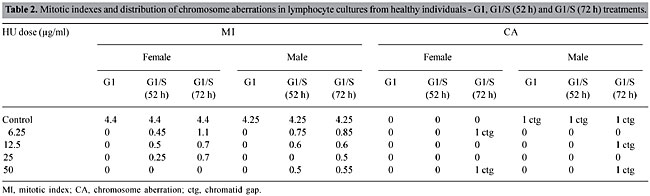
The microscope slide preparations were analyzed with an optical microscope to detect structural and numerical chromosome alterations in metaphases from the HU-treated cultures (Sanchez et al., 1991). The frequency of the chromosome aberrations (in 100 metaphases per culture) and the mitotic index (MI) (number of metaphases per 2,000 lymphoblasts per culture) were determined. Statistical analysis The Student t-test (Ayres et al., 2000a) was used to compare the frequencies of aberrations in cells exposed to the various dosages of HU, compared to the respective controls. The F-test (ANOVA) (Ayres et al., 2000b) was used to detect significant differences in the MI between cells exposed to HU, and the respective controls. The alpha level for statistical significance was 5%. RESULTS AND DISCUSSION Short-term cultures of phytohemagglutinin-stimulated peripheral blood lymphocytes were prepared in order to study the genotoxic effects induced by HU as well as its effects on MI. When HU was applied together with phytohemagglutinin to 72 h-cultures (G1 phase), there was a significant increase in cytotoxicity (P < 0.05) (Table 2), and no metaphases were found among the 2,000 cells. This finding means that HU is highly cytotoxic when the lymphocytes are stimulated by this mitogen. During blastogenesis, the lymphocyte is more sensitive than it is at other phases of the cell cycle. The genotoxic effect of HU was not significant at the tested doses (P > 0.5) (Table 2).
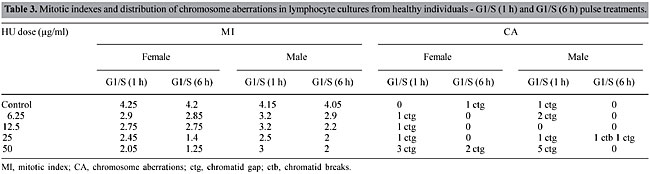
Cultures treated after 24 h of stimulation (G1-S phase) had a strong reduction in the MI (P < 0.05) (Table 2). This is as would be expected, considering that HU is a non-alkylating myelosuppressive substance that inhibits DNA synthesis through the inhibition of ribonucleotide reductase (Yarbro, 1992). Cells fixed at 52 h had a lower mitotic index than those fixed at 72 h. This is because at 52 h most cells had gone through one cell division; while at 72 h 70-75% of the cells were at the second or third division (Janossy and Greaves, 1972; Preston et al., 1987). Genotoxic effects were not significant at this phase (P > 0.5) (Table 2). In the pulse treatments (S phase), in which the cells were exposed to HU for 1 or 6 h, the MI was significantly decreased (P < 0.05, Table 3), though not as much as at 24 h. The 6-h pulse of HU was more cytotoxic than the 1-h pulse, showing that there is a time-dependent effect of HU exposure on MI. Exposition to HU at a dose of 50 µg/ml for 6 h gave an increased frequency of chromosome gaps, and the frequency of chromosome breaks was greatest at 25 µg/ml. However, these differences were not significant when compared to the control (P > 0.05) (Table 3).
Treatment with HU at the G2 phase reduced the MI significantly (P < 0.05) at all doses, except for the lowest dose (6.25 µg/ml, Table 4). This is due to the fact that this drug acts at the transition from G1 to S, though it also acts at G2, decreasing the MI, probably because it chemically contaminates the medium. This probable chemical contamination was not observed in controls because HU was not added. A strong reduction in the MI was observed at 12.5 µg/ml (LD 50, half the mitotic index of the control). At a concentration of 50 µg/ml the MI of the cells exposed to HU was 1/3 of the MI of the control (Table 4). The MI did not change further at 100, 150 and 200 µg/ml, showing that the chemical influence of this pharmaceutical material on G2 stabilizes from 50 µg/ml on. G2 phase treatment did not significantly increase the frequency of chromosome aberrations (P > 0.05) (Table 4).
The frequency of chromosome alterations in healthy individuals was not significantly changed by treatment with HU in all the cell cycle phases tested; moreover, there were no significant differences in the reaction of the lymphocytes from the male versus the female donor (data not shown). A nonsignificant increase in the number of chromosome gaps was observed in the pulse treatment (S phase); however, this should not be considered a criterion for genotoxicity because the precise origin of gaps is unknown(Preston et al., 1987). Absence of significant evidence of genotoxicity, observed in our study, supports the findings by Hanft et al. (2000), who showed HU to be less mutagenic than other anti-neoplastic HbF-inducing chemical agents. We conclude that HU is an S-phase-specific cytotoxic drug. It provoked alterations in the MI in in vitro lymphocyte cultures, as would be expected based on its inhibitory action on the synthesis of deoxyribonucleotides. ACKNOWLEDGMENTS Research supported by UFPA and CAPES. REFERENCES Antunes, L.M.G. and Takahashi, C.S. (1998). Effect of high doses of vitamins C and E against chromosomal damage induced by doxorubicin in rat bone marrow cells. Mutat. Res. 419: 137-143. Ayres, M., Ayres Jr., M., Ayres, D.L. and Santos, A.S. (2000a). Uma amostra. In: BioEstat 2.0 Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas (Sociedade Civil Mamirauá, CNPq, eds.). Belém, Sociedade Civil Mamirauá, CNPq, Brasília, DF, Brazil, pp. 77-87. Ayres, M., Ayres Jr., M., Ayres, D.L. and Santos, A.S. (2000b). Duas amostras relacionadas. In: BioEstat 2.0 Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas (Sociedade Civil Mamirauá, CNPq, eds.). Belém, Sociedade Civil Mamirauá, CNPq, Brasília, DF, Brazil, pp. 107-115. Donehower, R.C. (1992).An overview of the clinical experience with hydroxyurea. Semin. Oncol. 19: 11-19. Hanft, V.N., Fruchtman, S.R., Pickens, C.V., Rosse, W.F., Howard, T.A. and Ware, R.E. (2000). Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood 95: 3589-3593. Janossy, G. and Greaves, M.F. (1972). Lymphocyte activation. II. Discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin. Exp. Immunol. 10: 525-536. Kennedy, B.J. (1992). The evolution of hydroxyurea therapy in chronic myelogenous leukemia. Semin. Oncol. 19: 21-26. Lindenhahn, M. and Schubert, I. (1983). On the origin of hydroxyurea-induced chromatid aberrations in G2 chromosomes with BrdUrd in only one of the sister chromatids. Mutat. Res. 108: 301-316. Moorhead, P.S., Nowell, P.C., Mellmam, W.J., Battips, D.M. and Hungerford, D.A. (1960). Chromosome preparations of leukocytes cultured from human peripheral blood. Exp. Cell. Res. 20: 613-616. Preston, R.J., San Sebastian, J.R. and McFee, A.F. (1987). The in vitro human lymphocyte assay for assessing the clastogenicity of chemical agents. Mutat. Res. 189: 175-183. Sanchez, P.S., Gomes, R.A., Takahashi, C.S. Rodrigues, M.A.R., Rabello-Gay, M.N., Ribeiro, L.R., Ferrari, I., Pereira, C.A.B., Varella-Garcia, M., Almeida, T.M.B., Andrade, H.H.R., Reguly, M.L. and Marques, E.K. (1991). Mutagênese. In: Mutagênese, Teratogênese e Carcinogênese (Rabello-Gay, M.N., Rodrigues, M.A.R. and Monteleone-Neto, R., eds.). Sociedade Brasileira de Genética, Ribeirão Preto, SP, Brazil, pp. 11-167. Yarbro, J.W. (1992). Mechanism of action of hydroxyurea. Semin. Oncol. 19: 1-10. |
|