ABSTRACT. Drosophila ananassae has a unique status among Drosophila species because of certain peculiarities in its genetic behavior. The most unusual feature of this species is its relatively high frequency of spontaneous male recombination. The results of studies on non-sexual behavior, such as phototactic responses, eclosion rhythm, and preferences for oviposition and pupation sites, lead us to suggest that this behavior is under polygenic control, with a substantial amount of additive genetic variation. Sexual isolation has been reported in D. ananassae with the degree of such isolation being stronger in isofemale lines than in natural populations. The significant variations seen in the mating propensity of several isofemale strains, inversion karyotypes and wild type strains, the diminishing effects of certain mutations on the sexual activity of males, and the positive responses to selection for high and low mating propensity point to a genetic control of sexual behavior in D. ananassae. Males contribute more to variation and thus are more subject to intrasexual selection than females. There is a positive correlation between sternopleural bristle number, mating propensity and fertility in D. ananassae. This correlation between morphometric traits and mating success suggests that larger flies are more successful in mating than smaller ones. There is also evidence for adaptive plasticity and a trade-off between longevity and productivity in D. ananassae. Rare, specific courtship song parameters that provide males with a mating advantage have also been reported in different geographic strains of D. ananassae. The remating behavior of males and females, sperm displacement, and the bi-directional selection for female remating speed indicate that post-mating behavior in this species may also be under genetic control. The occurrence of size assortative mating further indicates that there is size-dependent sexual selection in D. ananassae. Key words: Drosophila ananassae, Behavior, Ethological isolation, Non-sexual behavior, Sexual behavior, Selection INTRODUCTION Behavioral genetics is concerned with the genetic analysis of individual differences in behavioral traits. These differences may occur naturally or may be induced through mutations. Naturally occurring variation forms the basis of traditional behavioral genetic approaches aimed at demonstrating a genetic basis for individual differences. However, induced behavioral mutations, which block or alter the normal pattern of behavior, provide a useful tool for understanding how genes influence behavior (Hall et al., 1982). Thus, behavioral genetics is the study of the hereditary control of an organism’s action. Through its behavior, an organism is able to obtain food, escape enemies, find a mate, and reproduce. Analysis of the genetic control of a given behavior is complicated by the fact that the primary action of the gene can affect: i) the sensory organs, thus changing information input, ii) an intermediary system (nervous, endocrine), thus altering coordination and perceptual capacities, and iii) the effector organ(s), thus altering the response. Behavioral genetics is a central discipline in the study of evolution. Caspari (1963) proposed that the genetic basis of behavioral characters is important because genes are the basic unit that is transmitted and reshuffled during evolution and are arranged by selective forces into patterns of adaptive action. A fundamental question in the study of the relationship between genes and behavior is whether heredity directly affects behavior or only defines the stage at which behavioral patterns may be molded by environmental factors. Indeed, the behavior of an individual develops in response to interactions between inherited limitations and environmental factors. The study of behavior is critically important for understanding speciation. Interspecies differences are effective in ensuring reproductive isolation in nature. As stated by Mayr (1963): “the shift into a new niche or adaptive zone is almost without exception, initiated by a change in behavior”. That is, there will initially be only minor changes at the structural level, and the evolution of morphological changes may follow behavioral changes. Some behaviors are regarded as innate, i.e., they are predominantly determined by the genotype. Such behaviors, which are based on genetic programs and do not allow appreciable modifications during translation into the phenotype, are known as closed programs. Other genetic programs are modified during translation into the phenotype by inputs occurring during the life span of the organism. They thus have an acquired component and are referred to as open programs. Closed programs are widespread in organisms with a short life span, including Drosophila, while open programs are more common in organisms that have longer life spans and show parental care. Mating is a good example of behavior in which the evolutionary significance of a closed program is particularly evident. Males generally display to females of their own species, and the females normally respond to such displays by accepting the courtship of these males based on an innate knowledge of their own species (Parsons, 1977). Drosophila ananassae, which was initially described from Indonesia by Doleschall in 1858, is a cosmopolitan and domestic species belonging to the ananassae species complex of the ananassae subgroup in the melanogaster species group (Bock and Wheeler, 1972). This species has been recorded from all six biogeographic zones and is common around human habitats (Sturtevant, 1942). Although cosmopolitan in distribution, D. ananassae is largely tropical. The first genetic investigations of D. ananassae were undertaken by Japanese workers in the 1930s. Kikkawa (1938) selected D. ananassae for genetic studies because of its excellent viability, high mutability, and certain peculiarities in its cytological and genetic behavior. It has since become clear that D. ananassae is unique among Drosophila species investigated thus far. D. ananassae has been extensively used for studies of population genetics, behavioral genetics, and recombination (Moriwaki and Tobari, 1975; Tobari, 1993; Singh, 1996), and about 500 papers have been published on genetic and biological aspects of this species (Singh, 1991, 2000; Tobari, 1993). Moriwaki writes: “In conclusion, I am happy to report that D. ananassae has secured a position as a unique and valuable organism for genetic research, especially characterized by male recombination and high mutability, both involving chromosomal and extra-chromosomal determinants, and by the Om-tom system” (in Tobari, 1993). The important peculiarities of D. ananassae include spontaneous meiotic male recombination, varied chromosomal polymorphism, high mutability, Y-4 linkage of the nucleolar organizer, segregation distortion, parthenogenesis, absence of genetic co-adaptation in different geographical populations, and extra-chromosomal inheritance (Tobari, 1993; Singh, 2000). An appreciable level of spontaneous crossing over in males of D. ananassae makes it a unique species within Drosophila (Kikkawa, 1938; Moriwaki, 1940; Moriwaki et al., 1970; Hinton, 1970; Moriwaki and Tobari, 1975; Matsuda et al., 1983; Singh and Singh, 1990; Mohanty and Singh, 1992). The optic morphology (Om) hypermutability system is unique in D. ananassae and there is an involvement of tom, a retrotransposon for the generation of Om mutations (Hinton, 1984; Matsubayashi et al., 1991; Awasaki et al., 1996). A spontaneous bilateral genetic mosaic, characterized by three mutant characters (cu, e, se-II chromosome) on the left side and all normal characters on the right side, was detected while scoring the progeny of a test cross between heterozygous males and mutant females (Singh and Mohanty, 1992). This is the first report of a spontaneous genetic mosaic for autosomal genes in Drosophila. This mosaic has been attributed to mitotic recombination in the zygote, which was genotypically heterozygous (Figure 1). A number of investigations on the sexual behavior, eclosion rhythm, preferences for oviposition and pupation sites, and phototactic behavior of D. ananassae have also been reported (Markow and Smith, 1979; Singh and Chatterjee, 1986, 1988a,b; Singh and Pandey, 1993a,b; Srivastava and Singh, 1997, 1998; Joshi, 1999; Singh and Singh, 1999a,b,c, 2000, 2001a,b,c; Doi et al., 2001; Yamada et al., 2002a,b; Sisodia and Singh, 2002, 2003).
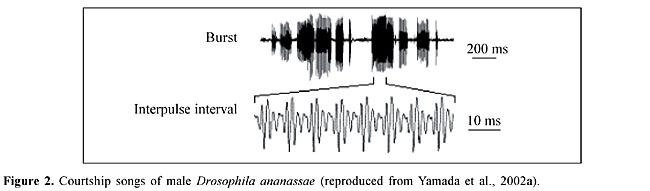
This review summarizes the genetic basis of the non-sexual and sexual behavior of D. ananassae which has been extensively investigated since Kikkawa (1938) used this species in his studies. In view of the uniqueness and usefulness of this species as a model organism for genetic studies, we provide a general historical overview of research with D. ananassae and discuss recent advances in our knowledge of this species’ biology. NON-SEXUAL BEHAVIOR The phototactic behavior of D. ananassae has been suggested to be under polygenic control and to be influenced by additive genetic variation (Markow and Smith, 1979). Latitudinal variation in the eclosion rhythm and in the locomotor activity rhythm has also been reported (Joshi, 1999; Joshi and Gore, 1999). The eclosion rhythm parameters of D. ananassae strains originating between 8° to 34° N are highly variable and latitude dependent. Under naturally fluctuating light intensity, temperature and relative humidity in the field, the amplitude of the rhythm is high and the eclosion gate is narrow. However, under a naturally fluctuating light intensity but at a constant temperature and relative humidity, the amplitude of the rhythm decreases and the width of the eclosion gate widens. When the eclosion rhythm is entrained to light-dark (LD) cycles ranging from LD 6:00-18:00 h to LD 18:00-6:00 h, the width of the eclosion gate decreases and increases in the short and long photoperiods, respectively. Among strains, the phase angle difference (psi, the time from lights-off in a 24-h LD cycle to the eclosion median) and the period of free-running rhythm (tau) in constant darkness vary by about 3 h, while the amplitude of the rhythmicity (Amp) varies by about 10%. A lower latitude correlates with a late psi, long tau and high Amp values. Khare et al. (2002) reported that the circadian pacemaker controlling the eclosion rhythm of the high altitude Himalayan strains of D. ananassae captured at Badrinath (5123 m) required an ambient temperature of 21°C for the entrainment and free-running processes. At this temperature, the eclosion rhythms entrained to 12-h LD (12:12) cycles and free-ran when transferred from constant light (LL) to constant darkness (DD), or upon transfer to a constant temperature (21°C) following entrainment to temperature cycles in DD. However, under identical experimental conditions, these strains were arrhythmic at 13° or 17°C. The eclosion medians always occurred in the thermophase of the temperature cycles, regardless of whether they were imposed in LL or DD, or whether the thermophase coincided with the photophase or scotophase of the concurrent 12:12-LD cycles. The temperature-dependent rhythmicity in Himalayan strains of D. ananassae is a rare phenotypic plasticity that may have been acquired through natural selection by accentuating the coupling-sensing mechanism of the pacemaker to temperature while simultaneously suppressing the effects of light on the pacemaker. Oviposition site preference is an important aspect of non-sexual behavior in adult female Drosophila. This behavior is closely related to fitness since pre-adult Drosophila have low mobility such that survival depends largely on the choice of the oviposition site by the female parents. D. ananassae females prefer the periphery of food vials for oviposition and tend to insert eggs into the medium (Srivastava and Singh, 1993a,b, 2001). Geographic strains of D. ananassae show variation with respect to oviposition site preference and there is a positive response to selection for the choice of oviposition site preference in D. ananassae (Srivastava and Singh, 1996b). The choice of oviposition site is influenced by extrinsic factors such as temperature, light and chemicals (Srivastava and Singh, 1996a, 1997, 1998). Pupation site preference is an important step in Drosophila pre-adult (larval) behavior because the site selected by larvae can have a decisive influence on their subsequent survival. This phenomenon has been studied in D. ananassae (Singh and Pandey, 1993a,b; Pandey and Singh, 1993). Although pupation height is influenced by various biotic and abiotic factors (Pandey and Singh, 1993), evidence for a genetic control of larval pupation behavior has been presented. The positive response to selection for high and low pupation height, and the intermediate pupation height of F1 hybrids produced by reciprocal crosses between high and low lines, show that pupation height in D. ananassae is under polygenic control (Singh and Pandey, 1993a). Crosses between two stocks with significant differences in pupation height have shown that the inheritance of pupation height fits a classic additive polygenic model, and that there is a substantial amount of additive genetic variation in natural populations of D. ananassae (Singh and Pandey, 1993b). Furthermore, the analysis of reciprocal backcrosses has shown a significant maternal effect. Progeny with low pupating mothers showed a lower pupation height than those with low pupating fathers, and progeny with high pupating mothers had a higher pupation height than those with high pupating fathers. Since the maternal effect was found only in backcrosses and not in the F1, this influence on pupation height follows the pattern of inheritance for a transient maternal effect (Singh and Pandey, 1993b). SEXUAL BEHAVIOR Since the sexual behavior of males and females affects and modifies the contribution of different genotypes to the gene pool of succeeding generations, such behavior is an important component of fitness. Sexual behavior must have evolved as an efficient mechanism to propagate the species. A variety of genotypes acting on the efficiency of sexual behavior at this focal stage would no doubt exert selection differences, and this will be of considerable value in perfecting the efficiency of the sexual process. In Drosophila, successful mating depends on male activity and female receptivity because the female is usually the discriminating partner in the mating act, i.e., she actively accepts or rejects a courting male (Ehrman and Parsons, 1981). Newly ecloded adult Drosophila show no sexual activity, and a species-specific period of time is necessary to reach sexual maturity. At maturity, adult flies can perform courtship, a behavioral ritual in which males and females exchange various types of acoustic, visual, chemical and tactile signals that culminate in copulation (Spieth and Ringo, 1983; Tomaru et al., 1998). The time elapsing from the beginning of courtship until copulation, generally referred to as the “mating speed” (or courtship time), is a good estimate of the sexual receptivity of females and of sexual activity in males (Spieth and Ringo, 1983). The male engages in a series of actions that include orienting towards and following the female, tapping her with his forelegs, singing a species-specific courtship song produced by extending one of his wings and vibrating it, licking the female’s genitalia, and curling his abdomen to attempt copulation (Spieth and Ringo, 1983). In most species, the forelegs, wings and mouth parts of the males serve as signaling structures. The only parts of the body that are not involved in courtship signaling in at least one or more species are the metathoracic legs. The female’s signals are fewer in number and diversity than those of the male and are produced by the wings, legs, genitalia and movements of the abdomen. These signals are produced in response to the courtship of a male and include rejection and acceptance responses (Spieth and Ringo, 1983). If the female has not recently mated and is sufficiently stimulated by the courtship of the male, she will slow down, open her vaginal plates, and allow copulation (Yamamoto et al., 1997). Males of D. ananassae court females by slightly spreading and vibrating both wings, and produce courtship songs by vibrating their wings intermittently during the sequence of courtship behavioral elements until copulation (Spieth, 1966). In addition, Yamada et al. (2002a) observed that male D. ananassae raised one or two legs (usually one leg) and shook them as if to display them to the female, most frequently immediately in front of her eyes. During this behavior, the shaken leg never touched the female. ETHOLOGICAL ISOLATION Sexual isolation has been considered one of the primary causes of speciation and its study has the potential to reveal the genetics of speciation. Spieth (1966) studied the mating behavior of light and dark forms of D. ananassae and found that various laboratory stocks developed sexual isolation, the extent of which varied from weak to marked. Sexual isolation between light and dark forms has been reported from Samoa (Futch, 1966). These forms were later found to be different sibling species (D. ananassae and D. pallidosa) of the ananassae complex. Although potentially interfertile, these sibling species are genetically distinct and their isolation is maintained by strong mating preferences (Futch, 1966). D. ananassae and D. pallidosa are useful for studying sexual isolation because of their sympatric distribution and because of the lack of post-mating isolation between them. Courtship songs play a crucial role in sexual isolation between these two species. Yamada et al. (2002a,b) reported that a very narrow region on the second chromosome was involved in controlling the female’s discriminatory behavior among courting males in D. ananassae. These authors also recorded and analyzed male courtship songs in several strains of D. ananassae and D. pallidosa and observed species specificity in the courtship song parameters (Yamada et al., 2002a). These parameters were suggested to play a role in mate recognition that enforced their sexual isolation. Bursts of male D. ananassae songs are polycyclic (Figure 2). Using surgical treatments, Doi et al. (2001) demonstrated that male courtship songs play a dominant role in female mate discrimination. The absence of a song in D. pallidosa dramatically increased interspecies mating with D. ananassae females but reduced intraspecies mating with D. pallidosa females. Furthermore, genetic analysis and chromosomal introgression by repeated backcrosses to D. pallidosa males identified possible loci that control the female discrimination in each species. These loci were mapped to distinct positions near the Delta locus in the middle of the left arm of the second chromosome.
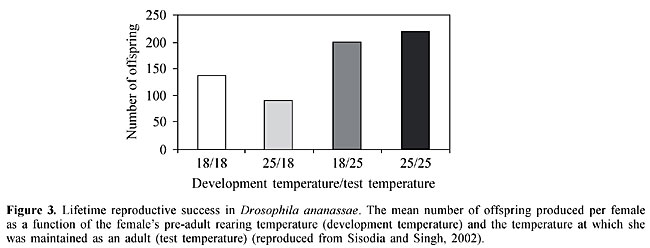
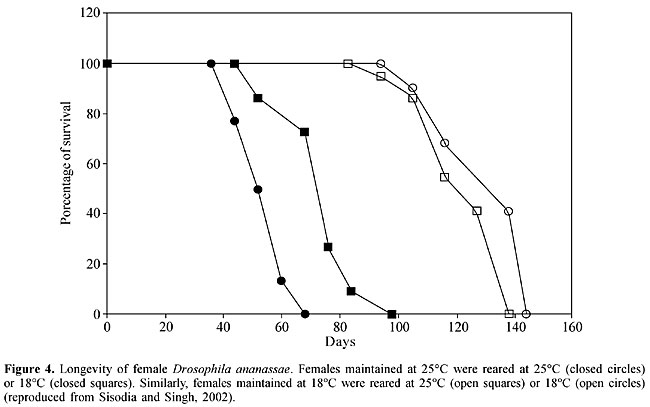
Because mate discrimination is well-developed and is the only known mechanism that prevents gene flow between species, these loci may have played crucial roles in the evolution of reproductive isolation and, therefore, in the speciation of these two species (Doi et al., 2001). Doi et al. (1997) identified the cuticular hydrocarbons (sex pheromones) in D. ananassae and D. pallidosa. (Z,Z)-5,27-tritriacontadiene and (Z,Z)-5,25-hentriacontadiene are major sex pheromone components in D. pallidosa and D. ananassae, respectively (Doi et al., 1997). Differences in the hydrocarbon or sex pheromone composition are important in mate recognition by males and probably represent one of the factors involved in the sexual isolation of the two species. Since F1 hybrid females from reciprocal crosses had half the amount of each of the sex pheromones in both species, the production of these substances is considered to be semi-dominant. An analysis of backcross females indicated that the third chromosome had a major influence on sex pheromone production in D. ananassae and D. pallidosa. To understand the genetic basis of sex pheromone perception in males, Doi et al. (1997) also analyzed courtship activity in F1 hybrids and backcross males. F1 hybrid males from reciprocal crosses courted both females and deployed with both sex pheromones. Chromosomal analysis showed that the third chromosome had a major effect on sex pheromone perception in both species. These findings indicate that sex pheromone production in females and perception in males are controlled by the same chromosome in D. ananassae and D. pallidosa. Singh and Chatterjee (1985a,b) reported sexual isolation in D. ananassae based on male choice experiments using isofemale lines and natural populations. In most of the crosses, homogamic matings outnumbered heterogamic ones, and the deviation from randomness was statistically significant. These findings provided evidence for positive assortative mating within D. ananassae. The degree of sexual isolation is stronger in isofemale lines than in natural populations and may involve genetic bottlenecks (Singh and Chatterjee, 1985b). Among the isofemale lines tested by Singh and Chatterjee (1985b), symmetrical and asymmetrical isolation was observed, with the latter being used to interpret the evolutionary sequences. These results clearly indicate that laboratory strains of D. ananassae have developed behavioral reproductive isolation as a result of genetic divergence. The findings with laboratory strains of D. ananassae are consistent with the hypothesis of Watanabe and Kawanishi (1979) that derived females discriminate against the ancestral males, a fundamental change for the creation of new species (Singh, 1997). MORPHOMETRIC TRAITS, MATING PROPENSITY AND FERTILITY The genetics of various quantitative traits have been studied in D. melanogaster. In particular, the number of sternopleural bristles has been widely used to assess the effect of artificial and natural selection, and to shed light on the genetic constitution of natural populations (see Singh and Mathew, 1996a, and references therein). Genetic heterogeneity for metrical characters such as sternopleural bristle number has been observed in Indian populations of D. ananassae (Singh and Mathew, 1996a). In addition, the selection for a high and low number of sternopleural bristles in D. ananassae has been found to correlate with the frequency of chromosomal inversions. A positive response to directional and stabilizing selection for sternopleural bristle number has been identified and provides evidence for the genetic control of sternopleural bristle phenotypes, with a substantial amount of additive genetic variation, in D. ananassae populations (Singh and Mathew, 1996a). Greater mating success was observed in D. ananassae flies with a high number of sternopleural bristles, which suggests that mating propensity in D. ananassae is influenced by sternopleural bristle phenotypes (Singh and Mathew, 1996b). Flies with a high number of sternopleural bristles showed greater fertility than those with a low number (Singh and Mathew, 1997). Thus, there is a positive correlation between sternopleural bristle number, mating propensity and fertility in D. ananassae. In D. melanogaster, Gibson and Thoday (1963) observed ethological isolation between flies with a high and low number of sternopleural bristles resulting from disruptive selection. The lines differing in bristle number developed sexual isolation as a consequence of genetic divergence. In contrast, Singh and Mathew (1996b) found no evidence of sexual isolation between high and low lines of D. ananassae that differed in bristle number. Mating propensity (success) is the result of a complex process in which behavioral, visual, acoustic, tactile and chemical communications occur between partners. The term mating propensity refers to the probability that an individual will mate within a given time interval. Mating propensity has been tested using mutant and wild-type isofemale strains of D. ananassae and the results confirm the genetic basis of this phenomenon (Chatterjee and Singh, 1987, 1988; Singh and Chatterjee, 1987). There is a positive correlation between mating propensity and fertility in D. ananassae (Singh and Chatterjee, 1987). Courtship time, duration of copulation and fertility have been tested in wild-type strains of D. ananassae, with significant variations being detected among them (Singh and Singh, 1999c). The strains with a longer copulation produced more progeny, indicating that there was a positive correlation between the duration of copulation and fertility in D. ananassae. Mating propensity in D. ananassae was tested using several wild-type strains. The mean number of matings varied significantly among the stocks tested and was attributable to the genetic heterogeneity among the stocks. Diallele analysis showed that the variation among the strains resulted from differences in the sexual activity of both sexes. However, the receptivity of females showed greater variation compared to the ability of males to mate (Singh and Singh, 1999b). Sisodia and Singh (2003) recently observed size assortative mating, which provides further evidence for sexual selection in D. ananassae. Chatterjee and Singh (1988) studied the effect of light and dark on the mating behavior of red- and white-eyed flies and found that red-eyed males were more successful in mating than white-eyed males in the light, and that white-eyed males were more successful in mating in the dark than in the light. The mating deficit of white-eyed mutants may be due to neurobehavioral disruption caused by faulty visual input. A significant decrease in the mating success of red-eyed males in the dark was also observed and probably reflected their lowered locomotor activity, thus indicating the importance of visual cues during courtship behavior in D. ananassae. To test the relationship between inversions and mating propensity, Singh and Chatterjee (1986, 1988a) studied the mating ability of homo- and heterokaryotypes. The latter originated from a subterminal (2L) inversion derived from natural populations of D. ananassae in which the frequencies of different chromosomal arrangements were known. The main conclusions of these studies were that: a) the chromosome occurring at a higher frequency was associated with higher mating activity, b) heterokaryotypic males were superior in mating propensity than homokaryotypes, indicating the existence of heterosis associated with the AL (alpha) inversion and male mating ability, and c) males showed greater variation than females, i.e., there were important sex-related differences. Thus, inversion polymorphism in D. ananassae may have a partial behavioral basis, as has been demonstrated in other Drosophila species. The polygenic control of mating activity has been demonstrated in D. ananassae based on the positive response to selection for high and low mating propensity (Singh and Chatterjee, 1988b). Males are much more affected by selection than females. The significant difference in mating activity of hybrids produced by the fast and slow males indicates the possibility of a Y-linked influence on mating activity in D. ananassae. When red-eyed males were tested separately with sepia and cardinal mutants at nine different ratios, both types of males were equally successful in mating when present in the same ratio, but each was more successful in mating than its counterpart when present as a minority. This advantage disappeared when the males became common. These observations provide evidence for a rare-male mating advantage in D. ananassae, and is a good example of frequency-dependent selection (Singh and Chatterjee, 1989). However, using female choice and multiple-choice techniques, Som and Singh (2001, 2002) found no evidence for a rare-male mating advantage in wild-type strains of D. ananassae. In these studies, experiments were done to study rare-male mating advantage in two karyotypically different strains of D. ananassae, ST/ST and AL/AL (2L). A significant, one-sided, rare-male mating advantage was observed in favor of AL males. The same data were used to compare the number of homogamic and heterogamic matings. Homogamic matings were significantly more frequent than heterogamic ones and the isolation estimate remained low (0.55-0.62), indicating preferential mating between females and males of the same karyotype. These findings provide evidence for a one-sided, rare-male mating advantage in favor of AL males, as well as sexual isolation between karyotypically different strains of D. ananassae (Singh and Som, 2001). Temperature is perhaps the most significant climatic parameter for explaining the geographic distribution of ectothermic species such as insects, especially since it plays a major role in ecogeographical differentiation. The body dimensions of poikilothermic animals are particularly affected since body dimensions increase when temperature decreases, although organisms such as Drosophila show sufficient behavioral plasticity to buffer the effect of changes in climate. The term phenotypic plasticity is used to describe the ability of an organism to alter its physiology, morphology or development in response to changes in its environment. Sisodia and Singh (2001) found a correlation between morphometric traits and mating success in D. ananassae and suggested that larger flies were more successful in mating than smaller flies. These authors also found evidence for adaptive plasticity and a trade-off between longevity and productivity in D. ananassae (Sisodia and Singh, 2002). The genetic response of body size to temperature in the laboratory provides an interesting example of phenotypic plasticity. Sisodia and Singh (2002) found that females of D. ananassae reared to adulthood at 18°C showed a significant increase in body weight compared to females reared at 25°C. At a given temperature, early productivity and lifetime productivity were highest when the rearing and test temperatures were the same (Figure 3). The effect of test temperature on total productivity and early productivity was highly significant, whereas that of development temperature was not. The interaction between test temperature and development temperature was also highly significant. Females reared at 18°C showed greater body weight but their productivity was not significantly greater than for smaller females reared at 25°C. Thus, the usually close relationship between size and fecundity was lost when the size change was due to rearing temperature. For a given test temperature, the flies reared at a different temperature were the longest lived (Figure 4). These findings provide evidence for adaptive plasticity in D. ananassae. Sisodia and Singh (2002) also found a negative correlation (trade-off) between longevity and productivity, the first report of such a trade-off in D. ananassae.
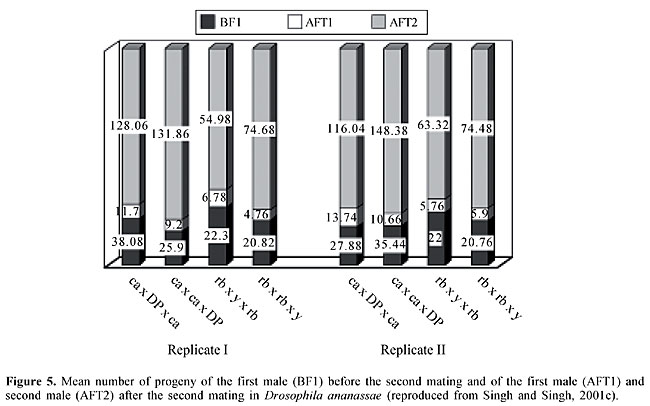
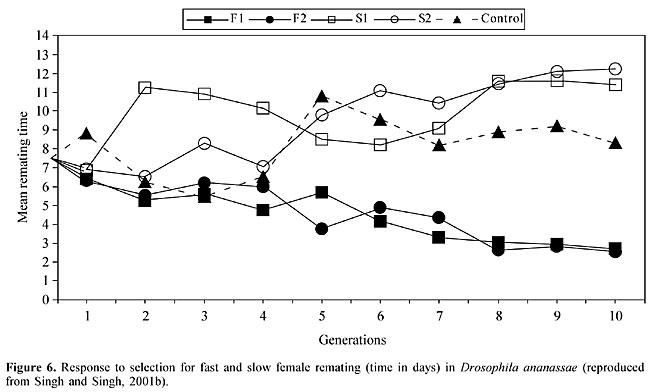
REMATING BEHAVIOR, SPERM DISPLACEMENT AND SEXUAL SELECTION The fact that males can mate with more than one female provides one of the bases for sexual selection, especially since the females generally accept only a limited number of mates (Petit and Ehrman, 1969). According to Gromko (1992), multiple mating is believed to be advantageous for males, and the selection of males could produce a correlated response in females. Female remating is an important component of Drosophila mating systems (see Singh et al., 2002) because females can store sperm in paired spherical spermathecae and a single elongate tubular seminal receptacle and use them to fertilize eggs as they are laid. Once a virgin female Drosophila has mated, she is usually unwilling to accept another male for some time because of the behavioral and physiological changes that occur after mating, including decreased attractiveness to males, decreased receptivity to further mating, increased egg laying, the storage and use of sperm, and a decreased lifespan (Fuyama, 1995; see Singh et al., 2002 and references therein). Remating by females is a prerequisite for sperm competition between males. Sperm competition is a powerful source in sexual selection since it shapes an organism’s reproductive behavior, morphology and physiology (Parker, 1970; Simmons, 2001). Male and female remating has been observed in D. ananassae (Singh and Singh, 1997, 1999a, 2000), with the frequency of female remating ranging from 24 to 56% in different strains. There is significant variation in remating latency (days) among strains. Significant variation has also been found in the duration of copulation between the first and second matings. In D. ananassae, the duration of copulation is shorter in the second mating compared to the first mating. Male remating occurs at a high frequency and varies within narrow limits (84-96%) in different strains. Interestingly, the male remating time varies from 7.41 to 21.59 min in different strains and this variation is highly significant. Males copulate for a shorter time during the second mating. Thus, the results of male and female remating experiments have shown that: i) remating occurs more frequently in males than in females, ii) there is strain variation in the remating times for both males and females, and iii) the duration of copulation is shorter in the second mating than in the first. This is the first demonstration of interstrain variations in male remating times in Drosophila. The influence of density on female remating frequency was tested using different wild-type and mutant strains of D. ananassae. Two experimental designs, i.e., a 2-h daily observation and continuous confinement, were used. The remating frequency depended significantly on the fly density in all strains tested using both experimental designs, except for one wild-type strain (Bhutan), which showed no density dependence in the 2-h daily observation protocol. Thus, the frequency of female remating in D. ananassae is enhanced at greater fly densities (Singh and Singh, 2001a). The effect of female remating on productivity and sperm displacement in D. ananassae was studied using different mutant strains and a wild-type strain. A comparison of once-mated (control) and remated females showed that in all of the crosses the productivity of remated females was significantly greater than that of females that mated only once. The P2' values (the proportion of second male progeny produced after remating) were calculated to assess sperm displacement in each cross of remated females. In all crosses, high P2' values (0.91-0.94) were observed, which suggested the occurrence of sperm displacement (Figure 5). In addition, female productivity increased after remating (Singh and Singh, 2001). Artificial selection for fast and slow remating speeds resulted in a rapid divergence in the remating times of both fast and slow lines (Figure 6) (Singh and Singh, 2001b). There were significant differences in the mean remating times of females from fast, slow, and control lines. The realized heritability over 10 generations of selection ranged from 0.26 to 0.33 for two replicates of the fast line and from 0.23 to 0.27 for two replicates of the slow line. These findings suggest that the female remating time in D. ananassae is under polygenic control. The remating frequency of females showed a correlated response in both fast and slow lines. By the tenth generation, the fast line flies were more successful in mating than slow and control line flies. The productivity of once-mated females, measured in terms of the number of progeny produced per female, showed that fast line females had more progeny than their slow and control line counterparts. Thus, fast lines had a positive effect on productivity and initial mating speed, while for slow lines the opposite was true. Selection experiments for fast and slow remating speed, and other studies, have shown that the remating speed, mating propensity, and fertility are all under polygenic control. The positive correlation between the duration of copulation and fertility in D. ananassae probably reflects the fact that the duration of copulation is an expression of the rate of sperm transfer (Singh and Singh, 1999a).
PERSPECTIVES Since the initial investigation by Kikkawa (1938), numerous studies have confirmed the uniqueness and usefulness of D. ananassae as a model organism for genetic studies. The various aspects of the behavioral genetics of D. ananassae discussed in this review have indicated several areas for future research. First, there is a need for a study of how the non-sexual behavior reported here is correlated with sexual behavior. Such experiments could assess whether there are any significant differences in the mating activity of flies showing phototactic behavior. Additionally, the relationship between light wavelength (UV and monochromatic) and mating activity, fecundity, remating ability and lifespan needs to be investigated. Second, the role of eclosion rhythm and locomotor activity in the mating ability and life span of these flies needs to be determined. Third, there is a need to assess a) whether oviposition or pupation site preferences confer any direct advantage to these flies in their ability to cope with different environmental conditions, b) whether oviposition at the periphery confers any survival advantage to the resulting progeny and the extent to which this is important for survival of the species, and c) whether pH influences the preference for oviposition and pupation sites. Finally, various studies that have examined the sexual behavior of this species have raised important questions that remain to be addressed, including the relationship between male recombination frequency and mating success, the influence of stress on mating ability, the influence of age and experience on mating activity, fluctuating asymmetry and mating success, the influence of mating on egg production and mortality in females, the role of transposable DNA elements and life history traits, the phenotypic and genotypic correlation of mating ability involving several candidate genes, the role of male accessory gland fluid in sperm management and in the life span of females, the effect of mating on the life span of flies, the influence of multiple matings on natural populations, and the genetic basis for mating and post-mating behavior and longevity. Future investigations of these and other aspects of the behavioral genetics of D. ananassae will expand our knowledge of this interesting species. ACKNOWLEDGMENTS S.R. Singh thanks Prof. A. Korol (University of Haifa) for providing a BSF fellowship for travel and work in his laboratory. We thank an anonymous reviewer for correcting the English and for valuable suggestions on the manuscript. REFERENCES Awasaki, T., Juni, N. and Yoshida, K.M. (1996). An eye imaginal disc-specific transcriptional enhancer in the long terminal repeat of the tom retrotransposon is responsible for eye morphology mutations of Drosophila ananassae. Mol. Gen. Genet. 251: 161-166. Bock, I.R. and Wheeler, M.R. (1972). Drosophila melanogaster species group. Univ. Tex. Publ. 7213: 1-102. Caspari, E. (1963). Genes and the study of behavior. Am. Zool. 3: 97-100. Chatterjee, S. and Singh, B.N. (1987). Variation in mating behaviour of Beadex mutant and wild type Drosophila ananassae. Indian J. Exp. Biol. 25: 278-280. Chatterjee, S. and Singh, B.N. (1988). Effect of light and dark on mating behaviour of red eye and white eye Drosophila ananassae. Indian J. Exp. Biol. 26: 611-614. Doi, M., Nemoto, T., Nakanishi, H., Kuwahara, Y. and Oguma, Y. (1997). Behavioral response of males to major sex pheromone component, (Z,Z)-5,25-hentriacontadiene, of Drosophila ananassae females. J. Chem. Ecol. 23: 2067-2078. Doi, M., Matsuda, M., Tomaru, M., Matsubayashi, H. and Oguma, Y. (2001). A locus for female discrimination behavior causing sexual isolation in Drosophila. Proc. Natl. Acad. Sci. USA 98: 6714-6719. Doleschall, C.L. (1858). Derde bijdrage tot de kennis der dipteren fauna van Nederlandsh Indie. Natuurk. Tijdschr. v. Ned. Indie 17: 73-128. Ehrman, L. and Parsons, P.A. (1981). Behaviour Genetics and Evolution. McGraw-Hill, New York, NY, USA. Futch, D.G. (1966). A study of speciation in South Pacific populations of Drosophila ananassae. Univ. Tex. Publ. 6615: 79-120. Fuyama, Y. (1995). Genetic evidence that ovulation reduces sexual receptivity in Drosophila melanogaster females. Behav. Genet. 25: 581-587. Gibson, J.B. and Thoday, J.M. (1963). Effects of disruptive selection. Heredity 18: 513-524. Gromko, M.H. (1992). Genetic correlation of male and female mating frequency: evidence from Drosophila melanogaster. Anim. Behav. 43: 176-177. Hall, J.C., Greenspan, R.J. and Harris, W.A. (1982). Genetic Neurobiology. MIT Press, Cambridge, MA, USA. Hinton, C.W. (1970). Identification of two loci controlling crossing over in males of Drosophila ananassae. Genetics 66: 663-676. Hinton, C.W. (1984). Morphogenetically specific mutability in Drosophila ananassae. Genetics 106: 631-653. Joshi, D.S. (1999). Latitudinal variation in locomotor activity rhythm in adult Drosophila ananassae. Can. J. Zool. 77: 865-870. Joshi, D.S. and Gore, A.P. (1999). Latitudinal variation in eclosion rhythm among strains of Drosophila ananassae. Indian J. Exp. Biol. 37: 718-724. Khare, P.V., Barnabas, R.J., Kanojiya, M., Kulkarni, A.D. and Joshi, D.S. (2002). Temperature dependent eclosion rhythmicity in the high altitude Himalayan strains of Drosophila ananassae. Chronobiol. Int. 19: 1041-1052. Kikkawa, H. (1938). Studies on the genetics and cytology of Drosophila ananassae. Genetica 20: 458-516. Markow, T.A. and Smith, L.D. (1979). Genetics of phototactic behaviour in Drosophila ananassae, a member of the melanogaster species group. Behav. Genet. 9: 61-67. Matsubayashi, H., Tobari, Y.N. and Hori, S.H. (1991). Genetic analysis of the Om (2D) locus in Drosophila ananassae. Jpn. J. Genet. 66: 387-397. Matsuda, M., Imai, H.T. and Tobari, Y.N. (1983). Cytogenetic analysis of recombination in males of Drosophila ananassae. Chromosoma 88: 286-292. Mayr, E. (1963). Animal Species and Evolution. Harvard University Press, Cambridge, MA, USA. Mohanty, S. and Singh, B.N. (1992). Effect of directional selection on spontaneous male recombination in Drosophila ananassae. Indian J. Exp. Biol. 30: 19-22. Moriwaki, D. (1940). Enhanced crossing over in the second chromosome of Drosophila ananassae. Jpn. J. Genet. 16: 37-48. Moriwaki, D. and Tobari, Y.N. (1975). Drosophila ananassae. In: Handbook of Genetics (King, R.C., ed.). Plenum Press, New York, NY, USA, pp. 513-535. Moriwaki, D., Tobari, Y.N. and Oguma, Y. (1970). Spontaneous crossing-over in the male of Drosophila ananassae. Jpn. J. Genet. 45: 411-420. Pandey, M.B. and Singh, B.N. (1993). Effect of biotic and abiotic factors on pupation height in four species of Drosophila. Indian J. Exp. Biol. 31: 912-917. Parker, G.A. (1970). Sperm competition and evolutionary consequences in insects. Biol. Rev. 45: 525-568. Parsons, P.A. (1977). Genes, behaviour and evolutionary processes: the genus Drosophila. Adv. Genet. 19: 1-32. Petit, C. and Ehrman, L. (1969). Sexual selection in Drosophila. In: Evolutionary Biology (Dobzhansky, T., Hecht, M.K. and Steere, W.C., eds.). Vol. 3. Appleton Century Croftz, New York, NY, USA, pp. 177-223. Simmons, L.W. (2001). Sperm Competition and its Evolutionary Consequences in the Insects. Princeton University Press, Princeton, NJ, USA. Singh, B.N. (1991). Bibliography on Drosophila ananassae. Drosophila Inf. Serv. 70: 206-211. Singh, B.N. (1996). Population and behaviour genetics of Drosophila ananassae. Genetica 97: 321-329. Singh, B.N. (1997). Mode of mating preference and the direction of evolution in Drosophila. Indian J. Exp. Biol. 35: 111-119. Singh, B.N. (2000). Drosophila ananassae - a species characterised by several unusual genetic features. Curr. Sci. 78: 391-398. Singh, B.N. and Chatterjee, S. (1985a). A study of sexual isolation among natural populations of Drosophila ananassae. Rev. Bras. Genet. VIII: 457-463. Singh, B.N. and Chatterjee, S. (1985b). Symmetrical and asymmetrical sexual isolation among laboratory strains of Drosophila ananassae. Can. J. Genet. Cytol. 27: 405-409. Singh, B.N. and Chatterjee, S. (1986). Mating ability of homo- and heterokaryotypes of Drosophila ananassae from natural populations. Heredity 57: 75-78. Singh, B.N. and Chatterjee, S. (1987). Variation in mating propensity and fertility in isofemale strains of Drosophila ananassae. Genetica 73: 237-242. Singh, B.N. and Chatterjee, S. (1988a). Parallelism between male mating propensity and chromosome arrangement frequency in natural populations of Drosophila ananassae. Heredity 60: 269-272. Singh, B.N. and Chatterjee, S. (1988b). Selection for high and low mating propensity in Drosophila ananassae. Behav. Genet. 18: 357-369. Singh, B.N. and Chatterjee, S. (1989). Rare male mating advantage in Drosophila ananassae. Genet. Sel. Evol. 21: 447-455. Singh, B.N. and Mathew, S. (1996a). Selection for high and low number of sternopleural bristles in Drosophila ananassae: correlated response in the frequency of chromosome inversions. Biol. Res. 29: 273-281. Singh, B.N. and Mathew, S. (1996b). Greater mating success of Drosophila ananassae flies possessing high number of sternopleural bristles. Curr. Sci. 70: 1088-1089. Singh, B.N. and Mathew, S. (1997). Greater fertility of Drosophila ananassae flies possessing high number of sternopleural bristles. Curr. Sci. 72: 112-114. Singh, B.N. and Mohanty, S. (1992). A spontaneous genetic mosaic in Drosophila ananassae. Curr. Sci. 62: 372-374. Singh, B.N. and Pandey, M.B. (1993a). Selection for high and low pupation height in Drosophila ananassae. Behav. Genet. 23: 239-243. Singh, B.N. and Pandey, M.B. (1993b). Evidence for additive polygenic control of pupation height in Drosophila ananassae. Hereditas 119: 111-116. Singh, B.N. and Singh, A.K. (1990). Linkage disequilibrium in laboratory strains of Drosophila ananassae is due to drift. Hereditas 112: 203-208. Singh, S.R. and Singh, B.N. (1997). Female remating in Drosophila ananassae. Drosophila Inf. Serv. 80: 38-39. Singh, B.N. and Singh, S.R. (1999a). Female remating in Drosophila ananassae: shorter duration of copulation during second mating as compared to first mating. J. Biosci. 24: 427-431. Singh, B.N. and Singh, S.R. (1999b). Mating success in Drosophila ananassae: evidence for greater variation in receptivity of females compared to male mating ability. Curr. Sci. 77: 1200-1203. Singh, S.R. and Singh, B.N. (1999c). Mating activity and fitness of a few wild type strains of Drosophila ananassae. Indian J. Exp. Biol. 37: 605-608. Singh, S.R. and Singh, B.N. (2000). Male remating in Drosophila ananassae: evidence for interstrain variation in remating time and shorter duration of copulation during second mating. Zool. Sci. 17: 389-393. Singh, S.R. and Singh, B.N. (2001a). Female remating in Drosophila ananassae: evidence for the effect of density on female remating frequency. J. Insect. Behav. 14: 659-668. Singh, S.R. and Singh, B.N. (2001b). Female remating in Drosophila ananassae: bidirectional selection for remating speed. Behav. Genet. 31: 361-370. Singh, B.N. and Singh, S.R. (2001c). Female remating in Drosophila ananassae: evidence for sperm displacement and greater productivity after remating. Zool. Sci. 18: 181-185. Singh, B.N. and Som, A. (2001). Evidence for rare male mating advantage and sexual isolation in two karyotypically different strains of Drosophila ananassae. Curr. Sci. 81: 1473-1477. Singh, S.R., Singh, B.N. and Hoenigsberg, H.F. (2002). Female remating, sperm competition and sexual selection in Drosophila. Genet. Mol. Res. 1: 178-215. Sisodia, S. and Singh, B.N. (2001). Mating success and morphometric traits in Drosophila ananassae. Curr. Sci. 80: 1444-1447. Sisodia, S. and Singh, B.N. (2002). Effect of temperature on longevity and productivity in Drosophila ananassae: evidence for adaptive plasticity and trade-off between longevity and productivity. Genetica 114: 95-102. Sisodia, S. and Singh, B.N. (2003). Size-dependent sexual selection in D. ananassae. Genetica (in press). Som, A. and Singh, B.N. (2001). Lack of evidence for rare male mating advantage in wild type strains of Drosophila ananassae. Curr. Sci. 81: 383-387. Som, A. and Singh, B.N. (2002). No evidence for minority male mating advantage in wild type strains of Drosophila ananassae tested in multiple-choice experiments. Genet. Mol. Res. 1: 317-326. Spieth, H.T. (1966). Mating behavior of Drosophila ananassae and ananassae-like flies from the Pacific. Univ. Tex. Publ. 3: 133-146. Spieth, H.T. and Ringo, J.N. (1983). Mating behavior and sexual isolation in Drosophila. In: The Genetics and Biology of Drosophila (Ashburner, M., Carson, H.L. and Thompson, J.N., eds.). Academic Press, London, England, pp. 223-284. Srivastava, T. and Singh, B.N. (1993a). Oviposition site preference in four species of Drosophila. Indian J. Exp. Biol. 31: 460-462. Srivastava, T. and Singh, B.N. (1993b). Intra-species variation with respect to oviposition site preference in certain Indian species of Drosophila. Evol. Biol. 7: 193-205. Srivasatava, T. and Singh, B.N. (1996a). Rhythmicity in oviposition pattern in light and darkness in four Indian species of Drosophila. Biol. Res. 29: 355-360. Srivastava, T. and Singh, B.N. (1996b). Bidirectional selection for choice of oviposition site in Drosophila ananassae. Korean J. Genet. 18: 295-300. Srivastava, T. and Singh, B.N. (1997). Effect of different chemicals on oviposition pattern in four Indian species of Drosophila. Braz. J. Biol. 57: 571-577. Srivastava, T. and Singh, B.N. (1998). Effect of temperature on oviposition in four species of the melanogaster group of Drosophila. Braz. J. Biol. 58: 489-493. Srivastava, T. and Singh, B.N. (2001). Choice of oviposition site between surface of the medium and paper in four Indian species of Drosophila. Indian J. Exp. Biol. 39: 383-386. Sturtevant, A.H. (1942). The classification of the genus Drosophila, with descriptions of nine new species. Univ. Tex. Publ. 4213: 5-51. Tobari, Y.N. (1993). Drosophila ananassae. Genetical and Biological Aspects. Japan Scientific Societies Press, Tokyo, Japan. Tomaru, M., Matsubayashi, H. and Oguma, Y. (1998). Effects of courtship song in interspecific crosses among the species of the Drosophila auraria complex (Diptera: Drosophilidae). J. Insect Behav. 11: 383-398. Watanabe, T.K. and Kawanishi, M. (1979). Mating preference and the direction of evolution in Drosophila. Science 205: 906-907. Yamada, H., Sakai, T., Tomaru, M., Doi, M., Matsuda, M. and Oguma, Y. (2002a). Search for species-specific mating signal in courtship songs of sympatric sibling species, Drosophila ananassae and D. pallidosa. Genes Genet. Syst. 77: 97-106. Yamada, H., Matsuda, M. and Oguma, Y. (2002b). Genetics of sexual isolation based on courtship song between two sympatric species: Drosophila ananassae and D. pallidosa. Genetica 116: 225-237. Yamamoto, D., Jallon, J.M. and Komatsu, A. (1997). Genetic dissection of sexual behavior in Drosophila melanogaster. Annu. Rev. Entomol. 42: 551-585. |
|