
ABSTRACT. Chromobacterium violaceum presents a distinctive phenotypic characteristic, the production of a deep violet pigment named violacein. Although the physiological function of this pigment is not well understood, the sequencing of the genome of this bacterium has given some insight into the mechanisms and control of violacein production. It was found that erythrose-4-phosphate (E4P), a precursor to aromatic amino acid biosynthesis, is produced by the non-oxidative portion of the hexose monophosphate pathway, since it lacks 6-phosphogluconate dehydrogenase. All genes leading from E4P plus phosphoenolpyruvate to tryptophan are present in the genome. Nevertheless, these genes are not organized in an operon, as in E. coli, indicating that other mechanisms are involved in expression. The sequencing data also indicated the presence and organization of an operon for violacein biosynthesis. Three of the four gene products of this operon presented similarity with nucleotide-dependent monooxygenases and one with a limiting enzyme polyketide synthase. As previously suggested, genes encoding proteins involved in quorum sensing control by N-hexanoyl-homoserine-lactone, an autoinducer signal molecule, are present in the bacterial genome. These data should help guide strategies to increase violacein biosynthesis, a potentially useful molecule. Key words: Chromobacterium violaceum, Tryptophan metabolism, Polyketide synthase, Tryptophan 2-monooxygenase, Violacein, N-acyl-homoserine synthase INTRODUCTION Since 1882, when Chromobacterium violaceum was first reported as an isolate from wet rice paste, its most notable characteristic has been the production of a deep violet pigment named violacein (Boisbaudran, 1882). The biological role of violacein in C. violaceum, as well as its biosynthesis pathway, has been under study since then. In early studies an increase in respiratory activity was observed when violacein extract was added to a non-pigmented cell suspension of C. violaceum, suggesting that violacein is a respiratory pigment (Friedheim, 1936). It was also suggested that violacein production is involved in the regulation of tryptophan production, which at high concentrations is toxic to bacteria (DeMoss, 1967). However, when grown on complex, complete medium, pigment production stops, showing that violacein is not required for C. violaceum growth and survival (Sivendra and Lo, 1975; Durán and Faljoni-Alario, 1980). The low solubility in water and the high molar extinction coefficient in methanol (e = 1.7 x 104 l mol-1 cm-1, l = 577 nm) lead us to suppose that violacein is involved in protection against visible radiation, since this bacterium is widely found in the water and soil of tropical and subtropical areas of the world. Although C. violaceum is able to grow under both aerobic and anaerobic conditions, violacein production occurs only in the presence of oxygen (DeMoss and Evans, 1959). DeMoss and Evans (1959) reported that in addition to violacein, C. violaceum produces a less abundant pigment named deoxyviolacein (DeMoss and Evans, 1959). The elucidation of the structure of violacein (3-[1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ylidene]-1,3-dihydro-2H-indol-2-one) began in 1958 through degradation and re-synthesis reactions of the compound (Ballantine et al., 1958). The data were confirmed by spectroscopic analysis in 1984 (Laatsch and Thomson, 1984). Several efforts have been made to determine the violacein biosynthesis pathway through studies on the role of tryptophan and other indol derivatives in the stimulation of violacein biosynthesis (DeMoss and Evans, 1960; Hoshino et al., 1987a,b; Hoshino and Ogasawara, 1990; Durán et al., 1994). Momen and Hoshino (2000) grew the bacteria on a mixture of [2-12C], [indole-3-13C] and [indole-2-13C] tryptophan, and found that all the carbon, hydrogen and nitrogen atoms of the violacein molecule come from tryptophan molecules. The hydroxylation of tryptophan with the production of an intermediate 5-hydroxytryptophan during the first steps of violacein biosynthesis suggested that molecular oxygen participates in an oxidation reaction in this synthesis, and confirmed that aerobic conditions are necessary for pigment production (Hoshino et al., 1987a,b; Hoshino and Ogasawara, 1990; Durán et al., 1994; Momen and Hoshino, 2000). The decarboxylation of one of the tryptophan molecules should occur later in the metabolic pathway, to yield violacein and related compounds (Durán et al., 1994; Momen and Hoshino, 2000). Since tryptophan appears to be the only precursor molecule in violacein biosynthesis, its production is apparently essential for pigment production in C. violaceum. The recently published sequencing data have confirmed several of the functional features of C. violaceum metabolism (Vasconcelos et al., 2003). RESULTS AND DISCUSSION Aromatic amino acid biosynthesis starts with erythrose-4-phosphate (E4P), an intermediate hexose monophosphate pathway (HMP), with phosphoenolpyruvate, a glycolysis pathway intermediate, leading through several steps to the production of chorismate, which can be directed to a branch that starts with anthranilate, leading to a tryptophan pathway. Surprisingly the HMP is incomplete in C. violaceum, since it lacks genes encoding for 6-phosphogluconate dehydrogenase, although all the other enzymes of the pathway seem to be present. This observation suggests that E4P, for tryptophan biosynthesis, is provided by the linkage between the intermediates of glycolysis, fructose-6-phosphate and glyceraldehyde-3-phosphate, through the activity of the transketolases and transaldolases of the nonoxidative steps of the HMP. This characteristic should enable C. violaceum to convert a larger amount of glucose to aromatic amino acids than other organisms that have a complete HMP. Ikeda and Katsumata (1999) showed that HMP-defective mutants produce an increased amount of E4P, which is the limiting substrate for tryptophan biosynthesis. As occurs in E. coli, tryptophan biosynthesis in C. violaceum starts with anthranilate synthesis; it is encoded by several genes (trpA, trpB, trpC, trpD, trpE, trpF, and trpG), but differently from E. coli, these genes are not organized into an operon (Table 1). However, they seem to compose clusters with genes not related to tryptophan biosynthesis. The entire operon for violacein biosynthesis has been cloned and sequenced (August et al., 2000). Their results were confirmed by the C. violaceum genome sequence, which showed that the biosynthesis genes are in an operon constituted of vioD, vioC, vioB, and vioA genes (Figure 1, Table 1). These authors also suggest a biosynthesis pathway with attributes of the activity of each gene product in the pathway (Figure 2). 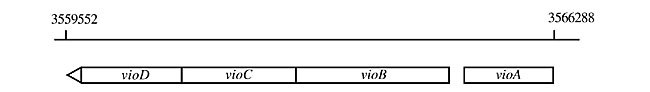
Figure 1. Schematic drawing of structural genes of the violacein biosynthesis operon.
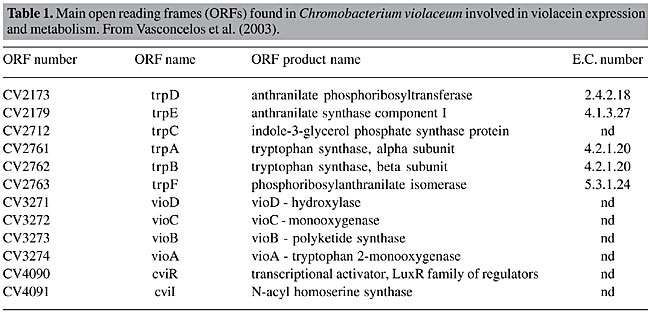
nd - not described.
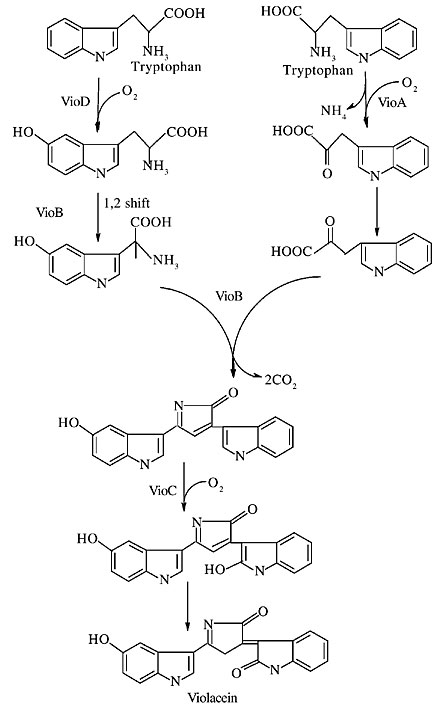
Figure 2. Violacein biosynthesis, as proposed by August et al., 2000. VioA, VioB, VioC, and VioD are the gene products of the biosynthesis operon, encoding nucleotide-dependent monooxygenases and a protein similar to a polyketide synthase (VioB).
The vioD, vioC and vioA products present similarity with nucleotide-dependent monooxygenases. While VioD seems to catalyze one tryptophan molecule hydroxylation, as suggested by most research on violacein biosynthesis, VioA catalyzes an oxidative deamination of a second tryptophan molecule, and VioC catalyzes intermediate violacein oxidation (August et al., 2000). The VioB protein is similar to polyketide synthase, an enzyme with a very interesting activity, since it is able to catalyze nonribosomal peptide bonds, and in violacein biosynthesis pathway it apparently catalyzes the condensation of two tryptophan derivative molecules, which are essential for pigment production (August et al., 2000). Violacein biosynthesis is under the control of a diffusible signal molecule, generically named N-acyl-homoserine-lactone (AHL); AHL mediates physiological responses in Gram-negative bacteria, including cell differentiation, production of secondary metabolites and exoenzymes (Chernin et al., 1998). An AHL identified and characterized as N-hexanoyl-homoserine-lactone (HHL) has been found in C. violaceum (McClean et al., 1997). Two genes cviI and cviR have been identified in the C. violaceum genome (Table 1), encoding CviI and CviR, HHL synthase and a regulator protein, respectively, with structural and functional similarity with chemicals that are involved in the control of bioluminescence in Vibrio fischeri. There apparently is a similar secondary metabolic control in C. violaceum. Possibly, when C. violaceum growth is near the stationary phase, a large amount of constitutive CviI leads to an accumulation of HHL. This signal molecule binds to the cviR operator, inducing its expression. The increase in CviR enables it to bind to a transcriptional regulator site of the violacein biosynthesis operon, increasing pigment production. CviR is able to stimulate the expression of some other genes in C. violaceum, such as chitinases and exoproteases (Chernin et al., 1998). On the other hand, HHL seems to be not the only control mechanism in C. violaceum production, since even in densely populated cell cultures, produced with high glucose concentration and oxygen levels, violacein production is inhibited (Oliveira, C.G., Porto, L.M. and Antônio, R.V., unpublished data). Violacein production is also dependent on the carbon source and the concentration in the culture medium. These experimental observations suggest that a control similar to that mediated by cyclic AMP, through catabolic activator protein, may mediate violacein biosynthesis. CONCLUDING REMARKS The sequencing of the C. violaceum genome shows that violacein biosynthesis is dependent on the metabolism of carbohydrates, not only through the HMP pathway, but also through the glycolysis and the Entner-Dourduroff pathways, from which NADPH should be produced, since this bacterium lacks 6-phosphogluconate activity. The similarity of three of the gene products of the operon of violacein biosynthesis to nucleotide-dependent monooxygenases, as well as the need for oxygen for pigment production, suggests that NADH or FADH2 are not such nucleotides, or they could be shared with violacein biosynthesis, since under aerobic conditions they should also be directed to the respiratory chain. The reducing power of violacein biosynthesis is not clear and should be one of the limitations for pigment biosynthesis. A quorum sensing system mediated by HHL is also present, and it seems to be very similar in organization to that found in Pseudomonas aeruginosa. ACKNOWLEDGMENTS The study described here was undertaken within the context of the Brazilian National Genome Program (a consortium funded in December 2000 by the Ministério da Ciência e Tecnologia (MCT) through the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). REFERENCES August, P.R., Grossman, T.H., Minor, C., Draper, M.P., MacNeil, I.A., Pemberton, J.M., Call, K.M., Holt, D. and Sosburne, M.S. (2000). Sequence analyses and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum. J. Mol. Microbiol. Biotechnol. 4: 513-519. Ballantine, J.A., Beer, R.J., Crutchley, D.J., Dodd, G.M. and Palmer, D.R. (1958). The synthesis of violacein and related compounds. Proc. Chem. Soc. 1: 232-234. Boisbaudran, L.D. (1882). Matiére colorante se formant dans la cole de farine. Compt. Rend. 94: 562. Chernin, L.S., Winson, M.K., Thompson, J.M., Haran, S., Bycroft, B.W., Chet, I., Willians, P. and Stewart, G.S.A.B. (1998). Chitinolytic activity in Chromobacterium violaceum: a substrate analysis and regulation by quorum sensing. J. Bacteriol. 180: 4435-4441. DeMoss, R.D. (1967). Violacein. Antibiotics 2: 77-80. DeMoss, R.D. and Evans, N.R. (1959). Physiological aspects of violacein biosynthesis in nonproliferating cells. J. Bacteriol. 78: 583-586. DeMoss, R.D. and Evans, N.R. (1960). Incorporation of C14-labeled substrates into violacein. J. Bacteriol. 79: 729-735. Durán, N. and Faljoni-Alario, A. (1980). Bacterial chemistry-I: Studies of a potential phototherapeutic substance from Chromobacterium violaceum. An. Acad. Bras. Cienc. 52: 297-301. Durán, N., Antônio, R.V., Haun, M. and Pilli, R.A. (1994). Biosynthesis of a trypanocide by Chromobacterium violaceum. World J. Microbiol. Biotechnol. 10: 686-690. Friedheim, E.A.H. (1936). La fonction respiratoire du pigment du Baccilus violaceus. Compt. Rend. Soc. Biol. 110: 352. Hoshino, T. and Ogasawara, N. (1990). Biosynthesis of violacein: evidence for the intermediary of 5-hydroxy-L-tryptophan and the structure of a new pigment, oxyviolacein, produced by the metabolism of 5-hydroxytryptophan. Agric. Biol. Chem. 64: 2339-2345. Hoshino, T., Kondo, T., Uchiyama, T. and Ogasawara, N. (1987a). Biosynthesis of violacein: a novel rearrangement in tryptophan metabolism with a 1,2-shift of the indole ring. Agric. Biol. Chem. 51: 965-970. Hoshino, T., Takano, T., Hori, S. and Ogaswara, N. (1987b). Biosynthesis of violacein: Origens of hydrogen, nitrogen and oxygen atoms in the 2-pyrrolidone nucleus. Agric. Biol. Chem. 51: 2733-2741. Ikeda, M. and Katsumata, R. (1999). Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl. Environ. Microbiol. 65: 2497-2502. Laatsch, H. and Thomson, R.H. (1984). Spectroscopic properties of violacein and related compounds: Crystal structure of tretramethylviolacein. J. Chem. Soc. Perkin Trans. II: 1331-1335. McClean, K.H., Winson, M.K., Fish, L., Taylor, A., Chhabra, S.R., Camara, M., Daykin, M., Lamb, J.H., Swift, S., Bycroft, B.W., Steward, G.S. and Williams, P. (1997). Quorum sensing in Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143: 3703-3711. Momen, A.Z. and Hoshino, T. (2000). Biosynthesis of violacein: intact incorporation of the tryptophan molecule on the oxindole side, with intramolecular rearrangement of the indole ring on the 5-hydroxyindole side. Biosci. Biotechnol. Biochem. 64: 539-549. Sivendra, R. and Lo, H.S. (1975). Identification of Chromobacterium violaceum: pigmented and nonpigmented strains. J. Gen. Microbiol. 90: 21-23. Vasconcelos, A.T.R., Almeida, D.F., Hungria, M., Guimarães, C.T., Antônio, R.V., Almeida, F.C., Almeida, L.G.P., Almeida, R., Alves-Gomes, J.A., Andrade, E.M., Araripe, J., Araujo, M.F.F., Astolfi-Filho, S., Azevedo, V., Baptista, A.J., Bataus, L.A.M., Baptista, J.S., Belo, A., van den Berg, C., Bogo, M., Bonatto, S., Bordignon, J., Brigido, M.M., Brito, C.A., Brocchi, M., Burity, H.A., Camargo, A.A., Cardoso, D.D.P., Carneiro, N.P., Carraro, D.M., Carvalho, C.M.B., Cascardo, J.C.M., Cavada, B.S., Chueire, L.M.O., Creczynski-Pasa, T.B., Cunha Junior, N.C., Fagundes, N., Falcão, C.L., Fantinatti, F., Farias, I.P., Felipe, M.S.S., Ferrari, L.P., Ferro, J.A., Ferro, M.I.T., Franco, G.R., Freitas, N.S.A., Furlan, L.R., Gazzinelli, R.T., Gomes, E.A., Gonçalves, P.R., Grangeiro, T.B., Grattapaglia, D., Grisard, E.C., Hanna, E.S., Jardim, S.N., Laurino, J., Leoi, L.C.T., Lima, L.F.A., Loureiro, M.F., Lyra, M.C.C.P., Madeira, H.M.F., Manfio, G.P., Maranhão, A.Q., Martins, W.S., Mauro, S.M.Z., Medeiros, S.R.B., Meissner, R.V., Moreira, M.A.M., Nascimento, F.F., Nicolas, M.F., Oliveria, J.G., Oliveira, S.C., Paixão, R.F.C., Parente, J.A., Pedrosa, F.O., Pena, S.D.J., Pereira, J.O., Pereira, M., Pinto, L.S.R.C., Pinto, L.S., Porto, J.I.R., Potrich, D.P., Ramalho Neto, C.E., Reis, A.M.M., Rigo, L.U., Rondinelli, E., Santos, E.B.P., Santos, F.R., Schneider, M.P.C., Seuanez, H.N., Silva, A.M.R., Silva, A.L.C., Silva, D.W., Silva, R., Simões, I.C., Simon, D., Soares, C.M.A., Soares, R.B.A., Souza, E.M., Souza, K.R.L., Souza, R.C., Steffens, M.B.R., Steindel, M., Teixeira, S.R., Urmenyi, T., Vettore, A., Wassem, R., Zaha, A. and Simpson, A.J.G. (2003). The complete genome of Chromobacterium violaceum reveals remarkable and exploitable bacterial adaptability. Proc. Natl. Acad. Sci. USA 100: 11660-11665. |
|