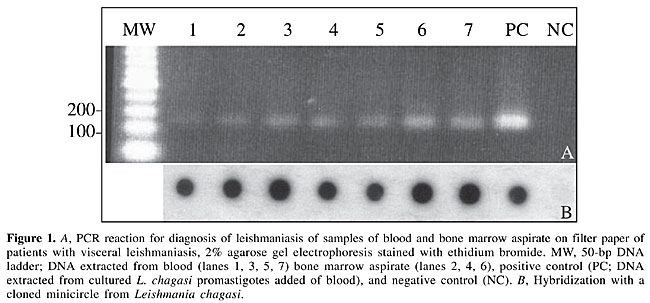
ABSTRACT. The polymerase chain reaction (PCR) is a simple, rapid procedure that has been adapted for the diagnosis of leishmaniasis. In the present study, 85 blood samples and seven bone marrow aspirates from 85 patients with clinical symptoms suggestive of visceral leishmaniasis from the metropolitan region of Belo Horizonte in the Brazilian State of Minas Gerais were screened using molecular and serological techniques. Samples that were negative (N = 12) and positive (N = 19) in parasitological and serological tests were used as controls. Of the 85 samples analyzed by PCR, 61 (71.7%) showed the expected amplification products in agarose gels. However, when the technique was combined with molecular hybridization, 72 samples (83.5%) gave a positive signal on film. Nineteen patients with Leishmania parasites in bone marrow cultures (positive controls) showed PCR hybridization in whole-blood samples, as did the seven bone marrow aspirates positive for Leishmania. None of the negative controls reacted in PCR or in an indirect immunofluorescent assay. These results indicate that PCR could replace the conventional parasitological examination in the diagnosis of leishmaniasis since it provides very satisfactory results with blood samples spotted on filter paper. Key words: Diagnosis, Filter paper, Leishmania chagasi, Serological analysis, Visceral leishmaniasis INTRODUCTION The etiological agents of visceral leishmaniasis (VL) are Leishmania chagasi (New World), L. infantum (Mediterranean region) and L. donovani (Indian subcontinent and Africa) (World Health Organization, 1990). Leishmaniasis is generally fatal if untreated (Meinecke et al., 1999) and its clinical symptoms include mainly intermittent fever, enlargement of the spleen, and pancytopenia. The diagnosis of leishmaniasis is based on clinical and epidemiological characteristics, on identification of the parasite during microscopic examination, or on indirect detection through serological tests. The serodiagnosis of leishmaniasis can be done using several methods. Indirect immunofluorescent assays (IFA) were the preferred method up to 1974 (Zuckerman, 1975). Since then, counter-current immunoelectrophoresis and enzyme-linked immunosorbent assays have proven to be useful in the diagnosis of this disease (Mukerji et al., 1984). However, these three methods have several limitations, including a low sensitivity and specificity. The development of the polymerase chain reaction (PCR) (Saiki et al., 1988) has led to the introduction of procedures for the detection and genetic characterization of Leishmania (Degrave et al., 1994). Since its introduction, PCR has been widely used to diagnose human and canine VL because of its high sensitivity and specificity (Ashford et al., 1990; Mathis and Deplazes, 1995; Berrahal et al., 1996; Osman et al., 1997; Roura et al., 1999; Campino et al., 2000; Silva et al., 2000, 2001a, 2002). PCR has also been used to diagnose VL in epidemiological studies and to detect the parasite in potential vectors and reservoirs. In addition, PCR can be used to investigate the phylogenetic relationships among Leishmania strains and species (Cupolillo et al., 2003). The increasing importance of VL in man and its high rate of lethality in the metropolitan region of Belo Horizonte in the Brazilian State of Minas Gerais (Silva et al., 2001b), mean that a rapid, relatively simple method is required for the routine diagnosis of VL. The aim of the present study was to standardized a PCR assay for the diagnosis of VL using blood and bone marrow samples spotted on filter paper. MATERIAL AND METHODS Patients Eighty-five patients with clinical symptoms of VL from areas around Belo Horizonte, Minas Gerais, were studied. Eighty-five blood samples and seven positive bone marrow aspirates from these patients were used in molecular and serological analyses. Blood from 12 volunteers from the State of Santa Catarina in southern Brazil (an area non-endemic for VL) served as negative controls, while blood samples from 19 patients with positive parasitological and serological tests, were used as positive controls. Serological test The IFA was done using a commercial kit for the diagnosis of human leishmaniasis (Fiocruz - Bio-Manguinhos) to detect antileishmanial antibodies in serum diluted from 1:40 to 1:640, according to the manufacturer’s instructions. Parasitological test Cells in bone marrow aspirates were cultured in NNN/LIT medium supplemented with 10% fetal calf serum and incubated at 24-25°C for 30 days. Parasites were detected in bone marrow cells by microscopic examination of Giemsa-stained slides. DNA isolation Samples (30 µl) of blood or bone marrow aspirates obtained from VL patients were spotted onto filter paper (FTA cards; Gibco BRL) and air-dried. The filter paper was then processed to remove enzymatic and cytotoxic contaminants prior to analysis (DNA remained bound to the paper during this solid phase clean-up). Following the initial clean-up, a small portion of the sample on FTA paper was removed using a 2-mm hole puncher and was placed in a microcentrifuge tube containing 200 µl lysis buffer (FTA processing reagent; Gibco BRL). The sample was then centrifuged three times (13,000 g, 5 min each) at room temperature and, after each centrifugation, the supernatant was removed prior to the addition of fresh buffer. After the third wash, 200 µl TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) was added to the microcentrifuge tube followed by incubation for 5 min at room temperature and centrifugation; the resulting supernatant was discarded. This procedure was repeated twice. The final paper pellet containing purified bound DNA was resuspended in 50 µl TE solution and used for PCR (Silva et al., 2002). Polymerase chain reaction The reactions were done in a total volume of 50 µl containing 200 µM of the dNTP mixture, 200 ng of each primer, 2.5 U of Taq polymerase (Perkin-Elmer, Branchburg, NJ, USA), and a small portion of the DNA paper sample in buffer. The PCR amplifications were done in a DNA thermocycler (Perkin-Elmer) using 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s, with a final extension at 72°C for 10 min. A hot-start PCR was done using the primers 5’(G/C)(G/C)(C/G)CC(A/C)CTAT(A/T)TTACACAACCCC3’ and 5’GGGGAGGGGCGTTCTGCGAA3’, derived from the conserved region of the minicircle DNA of the parasite kinetoplast (Degrave et al., 1994). Agarose gel electrophoresis Each experiment included a positive control (DNA from rabbit blood seeded with 106 promastigotes of L. chagasi strain MHOM/BR/74/PP75 per ml) and a negative control (DNA from rabbit blood without parasites). The 120-bp amplification products were analyzed by electrophoresis in polyacrylamide and agarose gels followed by silver staining and ethidium bromide staining, respectively. Southern blotting Following electrophoresis, the electrophoretic bands were transferred to a nylon membrane by capillary transfer and hybridized with cloned L. chagasi minicircles labeled with [a-32P]-deoxyadenosine triphosphate by random hexamer priming. The filters were hybridized at 65°C, washed in 0.1X sodium saline citrate at the same temperature, and exposed to X-ray film (Sambrook et al., 1989). RESULTS DNA purified from the positive controls (rabbit blood with L. chagasi promastigotes) yielded an amplification product of the expected size (120 bp) whereas DNA purified from non-contaminated rabbit blood was negative (Figure 1). No contamination or inhibition was detected in any of these samples.
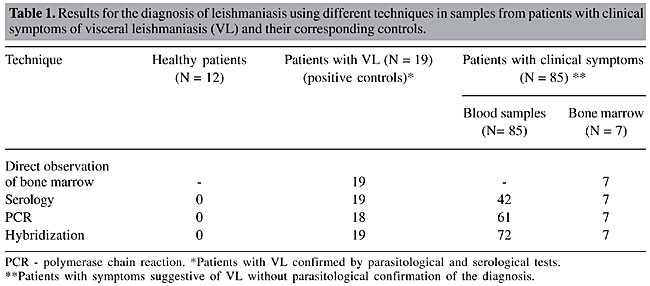
Of the 85 blood samples (42 seropositive and 43 seronegative) analyzed by PCR, 61 (71.7%) showed the expected product in agarose gels (Figure 1). However, when PCR was followed by Southern blotting, 11 samples that were negative by visual inspection of the agarose gels gave positive signals. All seropositive samples were also positive in the PCR hybridization test (Table 1).
Nineteen patients with parasites in their bone marrow cultures, considered as positive controls, were also positive when their blood samples were screened by PCR followed by hybridization. Similarly, the seven bone marrow aspirates were also positive. All negative controls (individuals from non-endemic areas with no clinical symptoms of VL and negative serology) were also negative by PCR. All of the PCR products were confirmed as L. chagasi after hybridization with a minicircle probe cloned from the same species (Figure 1). Table 1 compares the PCR results with the parasitological and serological tests. DISCUSSION Brazil accounts for more than 90% of the cases of VL registered in the Americas (World Health Organization, 1990). In recent years, VL has become a growing public health problem in various regions of this country, with the highest prevalence of the disease occurring in the northeastern region. Currently, the diagnosis of VL is based on clinical and epidemiological characteristics, parasitological analysis and the detection of anti-Leishmania antibodies. Confirmatory laboratory tests, which are rarely used, are restricted to a few reference centers and are not available to most patients. Direct microscopic identification of Leishmania is simple and cheap, but its sensitivity is very low, even when done by trained personnel (Mathis and Deplazes, 1995). Axenic culture is highly sensitive but requires special facilities, trained personnel, and is subject to contamination. Since some Leishmania strains are difficult to grow in the laboratory, parasite culture has been restricted to reference centers and used only for research purposes (Aviles et al., 1999). Serological tests are frequently used in epidemiological surveys of leishmaniasis and other parasitic diseases. One of the difficulties in defining the sensitivity and specificity of any test is the need for a gold standard that can correlate with the direct detection of the parasite (Dye et al., 1993). Immunological tests, such as IFA and ELISA show cross-reactivity with other diseases and do not distinguish between past and present infections. On the other hand, PCR, although considered highly sensitive, fails to distinguish viable parasites from degenerated ones. In addition, PCR products may be obtained from other kinetoplastids not normally expected to be found in humans (Smyth et al., 1992). In the present study, we used PCR with primers from the conserved region of Leishmania minicircles (Degrave et al., 1994) followed by hybridization with an L. chagasi probe to screen seropositive and seronegative individuals. This approch is very useful for diagnosing VL and can provide a better understanding of the epidemiology of visceral and cutaneous leishmaniasis in endemic areas (Passos et al., 1999; Silva et al., 2001b). Campino et al. (2000) used PCR to detect Leishmania DNA in peripheral blood obtained from HIV-positive patients with co-infection, but the species of Leishmania involved was not identified. Spotting samples onto filter paper allowed the material to be recovered from peripheral blood and simplified the extraction of DNA. In addition, once adsorbed to the filters, the material did not require storage at low temperature. The extraction protocol used was simple, rapid and did not require the use of organic solvents, thus making this procedure suitable for diagnosis purposes, even in areas lacking an adequate health service infrastructure. The ability of PCR to detect Leishmania in blood samples indicates that bone marrow aspirates are unnecessary for the diagnosis of VL and suggests that PCR could replace the conventional parasitological examination used clinically. The specificity and sensitivity of the PCR-based assay achieved using only a small quantity of blood spotted on filter paper makes the PCR assay the method of choice for patients suspected of having VL. ACKNOWLEDGMENTS The authors thank Dr. Edmundo Grissard for providing blood samples from volunteers in the State of Santa Catarina, Brazil. Research supported by PAPES/Fiocruz, CNPq, FUNASA (MS), TDR - OPAS, and PIBIC/Fiocruz. REFERENCES Ashford, D.A., Bozza, M., Freire, M., Miranda, J.C., Sherlock, I., Eulalio, C., Lopes, U., Fernandes, O., Degrave, W., Barker, R.H., Badaro, R. and David, J.R. (1990). Comparison of the PCR and serology for the detection of canine visceral leishmaniasis. Am. J. Trop. Med. Hyg. 53: 251-255. Aviles, H., Belli, A., Armijos, R., Monroy, F.P. and Harris, E. (1999). PCR detection and identification of Leishmania parasites in clinical specimens in Ecuador: a comparison with classical diagnostic methods. J. Parasitol. 85: 181-187. Berrahal, F., Mary, C. and Roze, M.S. (1996). Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am. J. Trop. Med. Hyg. 55: 273-277. Campino, L., Cortes, S., Pires, R., Oskam, L. and Abranches, P. (2000). Detection of Leishmania in immunocompromised patients using peripheral blood spots on filter paper and the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 19: 396-398. Cupolillo, E., Brahim, L.R., Toaldo, C.B., Oliveira-Neto, M.P., Brito, M.E.F., Falqueto, A., Naiff, M.F. and Grimaldi Jr., G. (2003). Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J. Clin. Microbiol. 41: 3126-3132. Degrave, W., Fernandes, O., Campbell, D., Bozza, M. and Lopes, U. (1994). Use of molecular probes and PCR for detection and typing of Leishmania - a mini review. Mem. Inst. Oswaldo Cruz 89: 463-469. Dye, C., Vidor, E. and Dereure, J. (1993). Serological diagnosis of leishmaniasis: on detecting infection as well as disease. Epidemiol. Infect. 110: 647-656. Mathis, A. and Deplazes, P. (1995). PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from humans and dogs. J. Clin. Microbiol. 33: 1145-1149. Meinecke, C.K., Schottelius, J., Oskam, L. and Fleischer, B. (1999). Congenital transmission of visceral leishmaniasis (Kala Azar) from an asymptomatic mother to her child. Pediatrics 104: e65. Mukerji, K., Roy, S., Mukhopadhyay, P., Gupta, P.K. and Ghosh, D.K. (1984). Evaluation of different subcellular fractions of Leishmania donovani for immunodiagnosis of visceral leishmaniasis. Indian J. Exp. Biol. 22: 120-122. Osman, O.F., Oskam, L. and Zijlstra, E.E. (1997). Evaluation of PCR for diagnosis of visceral leishmaniasis. J. Clin. Microbiol. 35: 2454-2457. Passos, V.M.A., Falcão, A.L. and Katz, N. (1999). Urban American cutaneous leishmaniasis in the metropolitan region of Belo Horizonte, Minas Gerais State, Brazil. Mem. Inst. Oswaldo Cruz 85: 243-244. Roura, X., Sanches, A. and Ferrer, L. (1999). Diagnosis of canine leishmaniasis by a PCR technique. Vet. Rec. 144: 262-264. Saiki, R.K., Gelfand, D.H. and Stoffel, S. (1988). Primer-direct enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487-491. Sambrook, J., Fritsh, E.F. and Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press, New York, NY, USA. Silva, E.S., Pirmez, C., Gontijo, C.M.F., Fernandes, O. and Brazil, R.P. (2000). Visceral leishmaniasis in the crab-eating fox (Cerdocyon thous) in south-east Brazil. Vet. Rec. 147: 421-422. Silva, E.S., Gontijo, C.M.F., Pirmez, C., Fernandes, O. and Brazil, R.P. (2001a). Short report: detection of Leishmania DNA by PCR on blood samples from dogs with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 65: 896-898. Silva, E.S., Gontijo, C.M.F., Pacheco, R.S., Fiuza, V.O.P. and Brazil, R.P. (2001b). Visceral leishmaniasis in the metropolitan region of Belo Horizonte, State of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 96: 285-291. Silva, E.S., Pacheco, R.S., Gontijo, C.M.F., Carvalho, I.R. and Brazil, R.P. (2002). Visceral leishmaniasis caused by Leishmania (Viannia) braziliensis in a patient infected with human immunodeficiency virus. Rev. Inst. Med. Trop. S. Paulo 44: 145-149. Smyth, A.J., Ghosh, A., Hassan, M.Q., Basu, D., De Bruijn, M.H., Adhya, S., Mallik, K.K. and Barker, D.C. (1992). Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala-azar patients. Parasitology 105: 183-192. World Health Organization (1990). Control of Leishmaniasis. Report of a WHO Expert Committee. Technical Report Series No. 793. World Health Organization, Geneva, Switzerland. Zuckerman, A. (1975). Current status of the immunology of blood and tissue Protozoa. I. Leishmania. Exp. Parasitol. 38: 370-400. |
|