
ABSTRACT. The cocoon, produced by most holometabolous insects, is built with silk that is usually produced by the larval salivary gland. Although this silk has been widely studied in the Lepidoptera, its composition and macromolecular arrangement remains unknown in the Hymenoptera. The macromolecular array patterns of the silk in the larval salivary gland of some meliponids, wasps, and ants were analyzed with polarized-light microscopy, and they were compared with those of Bombyx mori (Lepidoptera). There is a birefringent secretion in the glandular lumen of all larvae, due to filamentous structural proteins that display anisotropy. The silk in the distal, middle and proximal regions of the secretory portion of Formicidae and Vespidae glands presented a lattice optical pattern. We found a different pattern in the middle secretory portion of the Meliponini, with a zigzag rather than a lattice pattern. This indicates that the biopolymer fibers begin their macromolecular reorganization at this glandular region, different from the Formicidae and the Vespidae, in which the zigzag optical pattern was only found at the lateral duct. Probably, the mechanism of silk production in the Hymenoptera is a characteristic inherited from a common ancestor of Vespoidea and Sphecoidea; the alterations in the pattern observed in the Meliponini could be a derived characteristic in the Hymenoptera. We found no similarity in the macromolecular reorganization patterns of the silk between the Hymenoptera species and the silkworm. Key words: Polarized-light microscopy, Silk, Bee, Ant, Wasp, Silkworm, Silk gland, Larval salivary glands INTRODUCTION At the end of the larval period, a holometabolous insect will cease feeding and enter a quiescent state, known as pupation. Generally, pupae remain inside protective structures, or cocoons, which are usually built with material produced by the larval salivary gland. Bee salivary glands have a secretory portion, a lateral duct, and a common duct. In wasps, the morphology of the salivary gland is very similar; however, the lateral duct is longer and its secretory portion has two branches (Zara and Caetano, 2003). In ants, the salivary glands have a reservoir located between the secretory portion and the lateral ducts, and the secretory portion may have one or two branches. The ant Pachicondyla (=Neoponera) villosa has a secretory portion that appears to be interconnected by transverse commissures, and it ends in a loop, becoming a closed system. These characteristics differentiate the salivary gland of P. villosa from that of all the other Hymenoptera (Zara 1998, 2002; Zara and Caetano, 1999; Caetano et al., 2002). The morphological pattern is completely different in bees of the genus Bombus. The secretory portion is composed of several secretory branches, which have transverse commissures that open into a short lateral duct. This lateral duct is joined to another that stems from the other side of the body, forming the anterior duct (Flower and Kenchington, 1967; Mello and Vidal, 1979; Zara and Caetano, 2003). It is widely acknowledged that only the cells of the secretory portion are involved in the synthesis of the secretion, while the duct is the place where the secretion is usually modified (Cruz-Landim and Mello, 1969; Silva de Moraes and Cruz-Landim, 1975, 1979). The salivary gland secretion is silk, which has been widely studied in Bombyx for its protein composition and the configuration of its compounds (Akai and Kataoka, 1978; Matsumura, 1980; Minagawa, 1980). The silk fibers are composed of fibrous proteins, characterized by being water insoluble and quite strong (Rudall and Kenchington, 1971). In the Hymenoptera, the few studies that consider this subject have shown that their silk differs from the silk of the Lepidoptera in the following aspects: while in the Lepidoptera the secreted proteins present a b-sheet arrangement, with large amounts of glycine residues, the proteins secreted by the Hymenoptera show an a-helix arrangement, with fewer glycine and more acidic residues (Rudall, 1962; Flower and Kenchington, 1967; Lucas and Rudall, 1967; Rudall and Kenchington, 1971). The macromolecular pattern of silk can be quite variable among insects. At the ultrastructural level, regular and parallel arrangements of fibrous proteins have been observed in the lumen of the larval salivary glands of Apis, Vespa, and Bombus. These arrangements form the tactoids, which are highly birefringent under polarized light, thus indicating that they are anisotropic to the medium (Flower and Kenchington, 1967). Histophysical studies, using polarized-light microscopy, are useful to study the silk formation process in Arthropoda (Mello and Vidal, 1971; Silva, 1999; Silva-Zacarin et al., 2003; Jin and Kaplan, 2003). Although the composition of silk has been well studied, the degree of orientation and folding of their proteins, and the level of its macromolecular organization, are poorly understood. Rudall and Kenchington (1971) suggested that all fibrous proteins are phylogenetically homologous, but become diversified in their amino acid composition and macromolecular arrangement. The diversity in the amino acid composition of silk in arthropods was studied by Craig et al. (1999). We made an analysis of the macromolecular array pattern of the larval salivary gland secretion of some meliponids, wasps, and ants, using polarized light microscopy with compensating filters. These patterns, unknown until now for these species, were compared to each other and to the pattern in Bombyx mori. MATERIAL AND METHODS Material Silk glands of last-instar larvae of the following species were analyzed during the silk production period: Scaptotrigona postica, Melipona bicolor, Polistes versicolor, Polistes simillimus, Pachycondyla (=Neoponera) villosa, and Camponotus senex. The Hymenoptera specimens were collected in Rio Claro, SP, Brazil. The silkworms were obtained from sericulture in Charqueada, SP, Brazil. Methods The larvae were dissected in phosphate buffer (0.2 M, pH 7.4) and the isolated salivary glands were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The material was then dehydrated in a standard ethanol series (70 to 100%), washed in 100% xylol, transferred to slides and mounted in Entellan. These glands were analyzed under a polarized-light microscope, to detect the birefringence of the secretion. A compensating filter made of gypsum and mica (1st order red) was also used to evaluate the different deviations of polarized light in the secretion. RESULTS Scaptotrigona postica The silk gland secretion of S. postica larvae is birefringent throughout the gland’s extension. The secretion has an asymmetry in its crystalline structure, and it has the capability to rotate the plane of polarized light. In the posterior region of the secretory portion, the optical direction displays a lattice pattern (Figure 1A), with diverse deviations of polarized light, therefore producing diverse interference colors. On the other hand, in the middle secretory portion (Figure 1B), the biopolymer fibers appear organized, displaying a pattern with two colors of interference, forming a zigzag structure. In this pattern, the plane of polarized light is deviated in two optical directions: 45° left and 45° right of the plane of polarized light, when the compensating filter (1st order red = l/4) is used. In this type of pattern the change of direction is abrupt, which is reflected in the frequency of band alternation with different interference colors (Figure 1B). The zigzag pattern persists at the anterior secretory portion (Figure 1C) and in the ducts (Figure 1D), but when this zigzag pattern is compared to the one in the middle portion, the change in direction of the biopolymer is observed at larger intervals in the anterior portion (Figure 1C), while in the ducts there is low definition of the two-directional pattern (Figure 1D). In some regions of the lateral duct it is possible to visualize, at higher magnifications, a banding pattern that results from a differential birefringence, caused by the alternation of clear and dark bands (Figure 1E - arrows). 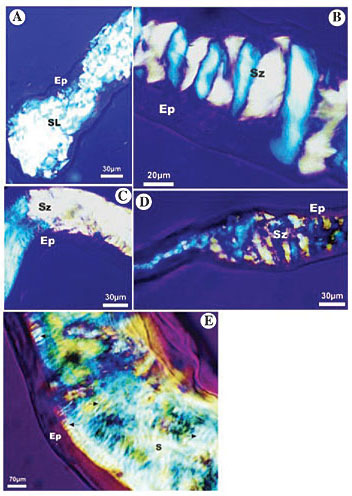
Figure 1. Photomicrography of larval salivary gland of Scaptotrigona postica analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in the gland lumen. A, The silk in the posterior region of the secretory portion shows a lattice pattern (SL); B, the silk in the middle region of the secretory portion shows a zigzag pattern (Sz); C, the silk in the anterior region of the secretory portion shows two directions (Sz); D, the duct has silk with a zigzag pattern (Sz), and E, lateral gland duct with a banding pattern, which is characterized by a differential birefringence (arrows). Ep = epithelium; S = secretion in the glandular lumen; SL = secretion in a lattice pattern; Sz = secretion in a zigzag pattern.
Melipona bicolor The silk present in the salivary glands of M. bicolor appears birefringent throughout the lumen, and the pattern of optical direction changes from the posterior to the middle region, passing from a lattice structure (Figure 2A) to a zigzag structure (Figure 2B). In the gland’s middle region the deviations of polarized light occur at longer intervals (Figure 2B) than the ones in the same glandular region of S. postica (Figure 1B). A pattern with a single optical direction was found in the anterior gland region (Figure 2C). A macromolecular reorganization of silk fibers was found in the duct, similar to the reorganization that occurs in the middle secretory region, although not as clear (Figure 2D). 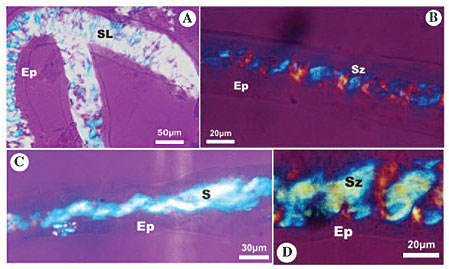
Figure 2. Photomicrography of larval salivary gland of Melipona bicolor, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in gland lumen. A, The silk in the posterior region of the secretory portion has a lattice pattern (SL); B, the silk in the middle region of the secretory portion shows a zigzag pattern (Sz); C, the silk in the anterior region of the secretory portion shows two directions (Sz), and D, the duct has silk with a zigzag pattern (Sz). Ep = epithelium; S = secretion in the glandular lumen; SL = secretion in lattice pattern; Sz = secretion in zigzag pattern.
Polistes versicolor The silk of P. versicolor larvae displays a lattice pattern of optical direction throughout the secretory portion (Figure 3A) and presents macromolecular reorganization in the lateral duct (Figure 3B), with a pattern of two well-defined optical directions forming the zigzag pattern. 
Figure 3. Larval salivary gland of Polistes versicolor, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in the gland lumen. A, The silk in the secretory portion shows a lattice pattern (SL) and B, the silk in the lateral duct shows a zigzag pattern (Sz). Ep = epithelium; SL = secretion in lattice pattern; Sz = secretion in zigzag pattern.
Polistes simillimus The birefringent secretion in the secretory portion of P. simillimus silk glands seems to have a two-optical direction pattern; however, the change in direction is not abrupt and occurs at well-spaced intervals (Figure 4A). In the lateral duct (Figure 4B) the reorganization of silk fibers shows patterns with two optical directions, exhibiting a slightly distended zigzag structure. 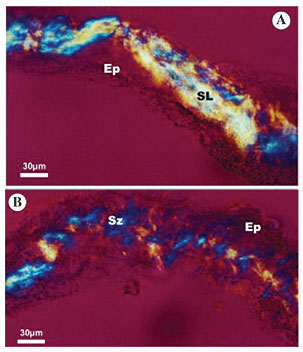
Figure 4. Photomicrography of larval salivary gland of Polistes simillimus, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in gland lumen. A, The silk in the secretory portion shows a lattice pattern (SL) and B, the silk in the lateral duct shows a zigzag pattern (Sz). EP = epithelium; SL = secretion in a lattice pattern; Sz = secretion in a zigzag pattern.
Pachycondyla (=Neoponera) villosa The secretory portion of the P. villosa salivary gland presents birefringent biopolymer fibers that show a lattice optical direction pattern (Figure 5A). This biopolymer appears compact; nevertheless, the birefringence is not maintained in the reservoir. The presence of a brown-colored secretion inside hampers the polarization of the light (Figure 5B). This pattern will only be altered when the biopolymer fibers reach the most proximal portion of the lateral duct, where two different optical directions (zigzag pattern) can be observed (Figure 5C). In addition, it can be noted that the inversion point of the polarized light plane is marked by a commissure (Figure 5C, arrow). This pattern is maintained, even when the biopolymer is found outside the epithelium of the gland’s lateral duct (Figure 5D, arrow). 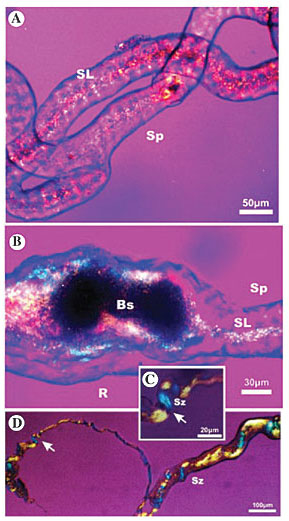
Figure 5. Photomicrography of larval salivary gland of Pachycondyla (=Neoponera) villosa, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in the gland lumen. A, The silk in the secretory portion has a lattice pattern (SL); B, the silk in the reservoir has a lattice pattern (SL) and there is some kind of brown coloration secretion (Bs); C, the silk in the most proximal portion of the lateral duct and the inversion point of the plane of polarized light (arrow), and D, the silk is found outside the epithelium of the lateral gland duct (arrow); it has a zigzag pattern (Sz). Ep = epithelium; Sp = secretory portion; R = reservoir; Bs = brown coloration secretion; SL = secretion in lattice pattern; Sz = secretion in zigzag pattern.
Camponotus senex The salivary gland secretion of C. senex has a strong birefringence throughout all glandular regions. The biopolymer found in the secretory region of the gland appears moderately compacted and shows a lattice pattern of optical direction (Figure 6A). This pattern is maintained in the gland reservoir; however, the silk found inside the reservoir is highly compacted (Figure 6B). This lattice pattern of optical direction is only altered when the silk passes from the reservoir to the lateral duct, where the biopolymer fibers change their reorganization from a lattice pattern to a two-optical direction pattern, and they display a zigzag structure (Figure 6C). 
Figure 6. Photomicrography of larval salivary gland of Camponotus senex, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in the gland lumen. A, The silk in the secretory portion has a lattice pattern (SL); B, the silk in the reservoir has a lattice pattern (SL), and C, the silk in the lateral duct shows a zigzag pattern (Sz). Sp = secretory portion; R = reservoir; SL = secretion in lattice pattern; Sz = secretion in zigzag pattern.
Bombyx mori At the lumen periphery in the secretory portion of B. mori, the secretion presents a pattern with two optical directions. However, at the lumen’s center, the secretion is isotropic (Figure 7A). The central secretion exhibits some organization (birefringence) in the anterior glandular region, while at the lumen’s periphery this region presents a secretion with a macromolecular organization in a single direction (Figure 7B and C - arrow). 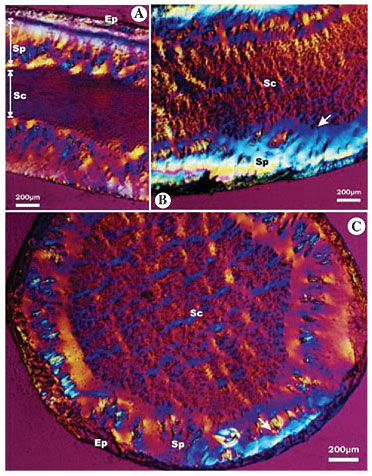
Figure 7. Photomicrography of larval salivary gland of Bombyx mori, analyzed under polarized microscopy with the first order compensating filter, showing the macromolecular array pattern of silk in the gland lumen. A, The silk at the lumen periphery (Sp) in the secretory portion shows two optical directions and the secretion at the center of the lumen (Sc) is isotropic; B, the secretion in the center of the lumen (Sc) in the anterior glandular region exhibits some organization and the secretion in periphery of the lumen (Sp) in the anterior glandular region has a macromolecular organization in a single direction (arrow), and C, transversal section of the anterior glandular region. The secretion in the center of the lumen (Sc) in the anterior glandular region exhibits some macromolecular organization and the secretion in periphery of the lumen (Sp) in the anterior glandular region shows a macromolecular organization in a single direction (arrow). Ep = epithelium; Sp = secretion in the periphery of the lumen; Sc = secretion in the center of the lumen.
DISCUSSION The salivary glands of last-instar larvae were found to produce a birefringent secretion when analyzed in whole mounts and under polarized-light microscopy. This indicates that the silk was already being compacted in the gland’s lumen. The distal regions of the secretory portion had a lattice pattern of optical direction under the plane of polarized light, characterized by several interference colors. However, in S. postica and M. bicolor, which belongs to the subfamily Apinae, tribe Meliponini (Michener, 1974, 2000), there was an alteration of the lattice pattern of optical direction in the gland secretion at the middle region of the secretory portion. In this region, the pattern of optical direction changes, forming a zigzag array. The functional significance of this event is that the biopolymer fibers begin to be reorganized at the macromolecular level in the glandular region. One of the differences found among the meliponine species was a single-optical direction pattern in M. bicolor and two-optical direction patterns in S. postica in the secretion of the anterior secretory portion of the salivary glands. The lattice pattern seen at the posterior secretory portion indicates the beginning of the macromolecular reorganization, which is completed at the middle portion, possibly due to the absorption of water by the epithelial cells (Oschman and Berridge, 1970; Silva de Moraes and Cruz-Landim, 1975; Silva, 1999) and to an increase in the compression of the secretion (Sehnal and Akai, 1990; Silva, 1999). These two events lead to an increase in the degree of silk compactation, which defines the pattern of two optical directions that result in the zigzag array. According to Silva-Zacarin et al. (2003), this pattern also results from silk compression in the lumen and indicates the high concentration of silk proteins, which are well oriented macromolecularly. In the anterior secretory portion of the salivary glands of S. postica and M. bicolor, the pattern of a single optical direction, or two directions at long intervals, is probably the result of silk release by the larva. This process would result in a partial loss of compactation, due to a reduction in the degree of compression. In Apis mellifera, the macromolecular reorganization of the silk to form a pattern of two optical directions, or zigzag array, occurs in the secretory portion, and it becomes better defined in the lateral duct (Silva, 1999; Silva-Zacarin et al., 2003). In other representatives of the subfamily Apinae, which have a common ancestor (such as in A. mellifera and Bombus lucorum), the primitively eusocial Bombini presents a pattern of reorganization of silk fibers very similar to the one found in the eusocial A. mellifera (Apini); this pattern being a little different from the one found in the highly eusocial Meliponini. Such differences in patterns of optical direction, added to distinct larval feeding strategies, could reflect different paths of natural selection in these tribes. The larvae of the tribes Bombini and Apini receive their food in a progressive manner (Michener, 1974, 2000), while the tribe Meliponini receive their meal in a mass, which is included in the group of behaviors known as POP (provision oviposition process) (Sakagami et al., 1965; Roubik, 1989; Zucchi, 1993; Velthuis, 1997). With regards to the optical pattern of silk production in the Formicidae, it has been previously mentioned that the secretory portion of the salivary gland presents the same lattice pattern of optical direction, which was clearly evidenced by the innumerous color interferences in P. villosa and C. senex. Nevertheless, the secretory portion of P. villosa clearly presents a smaller amount of material in the lumen, evidenced by the lower birefringence, in comparison with C. senex. Furthermore, in P. villosa, we noted an increase in birefringence in the reservoir. Nevertheless, another material of brownish color is added to the silk in this gland region. The addition of a brownish secretion was mentioned by Flower and Kenchington (1967), who speculated that this material is responsible for the brownish coloration seen in the silk of B. lucorum a few hours after the construction of the cocoon. This brownish secretion was not noticed in the reservoir of the salivary glands of C. senex. In both ant species studied, the pattern of optical direction in the reservoir had the same lattice pattern observed in the secretory portion. However, the absence of the brownish secretion in C. senex might be related to the fact that this ant produces a much larger amount of silk than P. villosa, or the difference could be due to the fact that these two species of ants are phylogenetically distant, since C. senex belongs to the Formicoid complex while P. villosa is found in the Poneroid complex (Hölldobler and Wilson, 1990; Gotwald Junior, 1995). The former proposition, concerning the presence of a larger amount of silk seems to be the most viable, since C. senex is known as the “weaver ant” and most of the silk produced by its larvae is used for the construction of its arboreous nest (Schremmer, 1979; Hölldobler and Wilson, 1977, 1990). On the other hand, the silk produced by the larvae of P. villosa is only used for the construction of the cocoon (Zara and Caetano, 1999, 2003; Zara 2002). The silk in both Formicidae studied present the same lattice pattern of optical direction in the distal portion of the lateral duct. However, the macromolecular arrangement of the biopolymer fibers begins in the lateral duct. The two optical directions begin to appear around this portion, displaying a zigzag pattern, similar to that observed in the secretory portion and in the lateral duct of A. mellifera (Silva-Zacarin et al., 2003), S. postica and M. bicolor. In the Vespidae, as in the Formicidae, the macromolecular array pattern in the production of silk had never been investigated. We studied two species of wasps that are considered primitively eusocial (Reeve, 1991): P. versicolor and P. simillimus. The macromolecular array pattern seen in these wasps was practically identical to the one found in ants, where the secretory portion presents a lattice pattern of optical directions. The main difference observed between wasps and ants concerns the morphology of the salivary gland, which in wasps lacks a reservoir and includes a long lateral duct (Zara and Caetano, 2003). Similar to the ants, a clear zigzag pattern can be observed in the anterior portion of the lateral duct of wasp salivary glands. This fact, which indicates an intense macromolecular reorganization of the silk in the duct, associates the histophysical characteristics of the silk with the morphological characteristics of the gland. The secretory portion in wasps and ants is branched, which could make it difficult to produce the zigzag pattern formation that is found in meliponids, which have an unbranched secretory portion of the silk gland (Zara and Caetano, 2003). The morphology of meliponini silk glands is similar to that of the A. mellifera silk gland, in which the zigzag pattern is more obvious in the duct than in the secretory portion (Silva-Zacarin et al., 2003). According to Silva-Zacarin et al. (2003), this difference in the zigzag pattern can be a function of gland morphology. Analysis of the Apis silk gland with scanning electron microscopy showed that the luminal secretion is in close contact with the epithelium of the secretory portion, but no contact was found between the secretion and duct epithelium, which allows the large cluster of compact fibrillar secretions to produce a spiral shape, giving it two optical directions. The patterns seen in the Formicidae were also found in the Vespidae, which in turn were very similar to those found in some Apidae. The similarities between Vespidae and Formicidae are easily explained when we take in consideration that both belong to the same phylogenetic branch, Vespoidea. On the other hand, the Apidae are found in another phylogenetical branch: the Sphecoidea (Brothers, 1975). The differences that were only seen in the silk formation in Meliponini could be yet another distinctive character, as behavior, colony structure, and post-embryonic development differ between the tribes Meliponini and Apini. Complementary studies using morphological, molecular and histological characters are necessary to test this hypothesis. Another aspect that distinguishes the Meliponini from the rest of the Hymenoptera is that their salivary gland probably only functions in silk production, since no protease activity was detected in salivary gland extracts of Scaptotrigona bipunctata larvae (Schumaker et al., 1993). On the other hand, the larval salivary glands of wasps present many functions besides the silk production. During post-embryonic development, these glands produce a large amount of secretion composed of sugars, proteases and free amino acids (Ishay and Ikan, 1968). The salivary glands of ants also have another important function: the production of digestive enzymes (Petralia et al., 1980; Zara, 2002). Thus, in both wasps and ants, the salivary glands have another function during post-embryonic development. This also seems to occur in Apis, but the functions of the salivary gland during post-embryonic development are not yet completely understood in this genus. Nevertheless, Silva (1999) speculates that another kind of secretion also occurs in Apis, which would approximate the function of the salivary glands of bees to that of wasps and ants, in addition to the similarities reported here concerning the silk production pattern. We believe that the processes of silk production in the Hymenoptera might be a characteristic inherited from a common ancestor of Vespoidea and Sphecoidea. Some alterations in the macromolecular array pattern observed in the tribe Meliponini, as well as the absence of silk production in the subfamilies Pseudomyrmicinae and Dolichoderinae of the Formicidae, would be derived characteristics within the Hymenoptera (Wheeler and Wheeler, 1976, 1979). In the Lepidoptera, the glandular secretion has already been well studied in B. mori, as regards protein composition and conformation of its components (Akai and Kataoka, 1978; Matsumura, 1980; Minagawa, 1980). Therefore, this species was used as an out-group in order to determine if the histophysical characteristics of silk fiber production in Lepidoptera and Hymenoptera are similar. In the posterior secretory portion of B. mori, there was an isotropic material at the center of the glandular lumen and an anisotropic material, with two optical directions at the margin. This observation agrees with the proposition made by Akai et al. (1987) and leads us to conclude that the secretion produced in the glandular lumen is originally isotropic; it would then become dehydrated and undergo subsequent macromolecular organization. This secretion corresponds to fibroin, which is released at the posterior portion in masses, known as spherical masses of fibroin fibers, and is surrounded by a liquid secretion (Akai and Kataoka, 1978). In the middle secretory portion, this secretion receives the sericin layers, which envelopes the fibroin fibers (Akai et al., 1987). The sericin, according to our observations, would correspond to the material with a pattern of a single optical direction observed at the periphery of the anterior region, and it appears to envelope the fibroin, which already exhibits a certain macromolecular array. In Hymenoptera, the silk is not organized in spherical masses of fibroin fiber. The silk of A. mellifera (Flower and Kenchington, 1967; Silva, 1999), Bombus sp. and Vespa sp. (Flower and Kenchington, 1967), P. villosa (Zara, 2002; Zara and Caetano, 2002) and C. senex (Zara and Caetano, personal communication) is composed of tactoids formed by the fusion of small amounts of secretion, which were released from the cells by exocytosis of the secretion vesicles that contain a fibrous material. This kind of secretion has been observed through transmission electron microscopy (Silva de Moraes and Cruz-Landim, 1979; Zara, 2002; Zara and Caetano, 2002; Silva-Zacarin et al., 2003). Although the silk secreted by Hymenoptera and Lepidoptera silk glands differs in its macromolecular array pattern, as shown in this study, Silva-Zacarin et al. (2003) speculated that the main steps involved in silk formation are similar in the two groups: polymerization of protein in the lumen forming typical structures (tactoids in A. mellifera and spherical mass of fibroin fibers in B. mori) and absorption of water from the spaces among these structures, along the gland, to form a well-organized and insoluble silk filament. REFERENCES Akai, H. and Kataoka, K. (1978). Fine structure of liquid fibroin in the posterior silkgland of Bombyx larvae. J. Seric. Sci. Jpn. 47: 273-278. Akai, H., Imai, T. and Tsubouchi, K. (1987). Fine-structural changes of liquid silk in silk gland during the spinning stage of Bombyx larvae. J. Seric. Sci. Jpn. 56: 131-137. Brothers, D.J. (1975). Phylogeny and Classification of the Aculeate Hymenoptera, with special reference to Mutllidae. Univ. Kans. Sci. Bull. 50: 483-648. Caetano, F.H., Jaffé, K. and Zara, F.H. (2002). Formigas: Biologia e Anatomia. Topázio, Araras, SP, Brazil. Craig, C.L., Hsu, M., Kaplan, D. and Pierce, N.E. (1999). A comparison of silk proteins produced by spiders and insects. Int. J. Biol. Macromol. 24: 109-118. Cruz-Landim, C. and Mello, M.L. (1969). The post-embryonic changes in Melipona quadrifasciata anthidioides Lep. (Hym., Apoidea). II. Development of the salivary glands system. J. Morphol. 123: 481-502. Flower, N.E. and Kenchington, W. (1967). Studies on insect fibrous proteins: the larval silk of Apis, Bombus and Vespa. J. R. Microsc. Soc. 86: 297-310. Gotwald Junior, W.H. (1995). Army Ants: The Biology of Social Predation. Cornell University Press, London, England. Hölldobler, B. and Wilson, E.O. (1977). Weaver ants. Sci. Am. 237: 146-154. Hölldobler, B. and Wilson, E.O. (1990). The Ants. Springer, London, England. Ishay, J. and Ikan, R. (1968). Food exchange between adults and larvae in Vespa orientalis F. Anim. Behav. 16: 298-303. Jin, H.J. and Kaplan, D.L. (2003). Mechanism of silk processing in insects and spiders. Nature 424: 1057-1061. Lucas, F. and Rudall, K.M. (1967). Extracellular fibrous proteins: the silks. In: Comprehensive Biochemistry 26B (Florkin, M. and Srots, E.H., eds.). Elsevier Publ., New York, NY, USA, pp. 475-558. Matsumura, H. (1980). Scanning electron microscopic observation of the fine structure of fibroin fibers. In: Struture of Silk Thread 2 (Hojo, J., ed.), Shinkyo Press, Nagano, Japan, pp. 175-185. Mello, M.L.S. and Vidal, B.C. (1971). Histochemical and histophysical aspects of silk secretion in Melipona quadrifasciata (Hym: Apoidea). Z. Zellforsch. Mikrosk. Anat 18: 555-569. Mello, M.L.S. and Vidal, B.C. (1979). The salivary gland secretion of a neotropical bumblebee. Protoplasma 100: 251-265. Michener, C.D. (1974). The Social Behavior of the Bees. Havard University Press, Cambridge, USA. Michener, C.D. (2000). The Bees of the World. Johns Hopkins, London, England. Minagawa, M. (1980). Fine structure and lousiness fibers. In: Structure of Silk Thread 2 (Hojo, J., ed.). Shinkyo Press, Nagano, Japan, pp. 187-208. Oschman, J.L. and Berridge, M. (1970). Structural and functional aspects of salivary secretion in Caliphora. Tiss. Cell 2: 281-310. Petralia, R.S., Sorensen, A.A. and Vinson, S.B. (1980). The labial gland system of larvae of the imported fire ant, Solenopsis invicta Buren. Cell Tiss. Res. 206: 145-156. Reeve, H.K. (1991). Polistes. In: The Social Biology of Wasps (Ross, K.G. and Matthews, R.W., eds). Cornell University Press, Ithaca, NY, USA, pp. 99-148. Roubik, D.W. (1989). Ecology and Natural History of Tropical Bees. Cambridge University Press, New York, NY, USA. Rudall, K.M. (1962). Silk and other cocoon proteins. In: Comparative Biochemistry 4 (Florkin, M. and Mason, H.S., eds.). Academic Press, New York, NY, USA, pp. 397-433. Rudall, K.M. and Kenchington, W. (1971). Arthropod silks: the problem of fibrous proteins in animal tissues. Annu. Rev. Entomol. 16: 73-96. Sakagami, S.F., Montenegro, M.J. and Kerr, W.E. (1965). Behavior studies of the stingless bees, with special reference of the oviposition process. V. Melipona quadrifasciata anthidioides Lepeletier. J. Fac. Sci. Hokkaido Univ. (Zool) 6: 578-607. Schremmer, V.F. (1979). Das Nest neotropischen Weberameise Camponotus (Myrmobrachys) senex Smith (Hymenoptera, Formicidae). Zool. Anz. 203: 273-282. Schumaker, T.T.S., Cristofoletti, P.T. and Terra, W.R. (1993). Properties and compartmentalization of digestive carbohydrases and proteases in Scaptotrigona bipunctata (Apidae: Meliponinae) larvae. Apidologie 24: 3-17. Sehnal, F. and Akai, H. (1990). Insect silk glands: their types, development and function, and effects of environmental factors and morphogenetic hormones on them. Int. J. Insect Morphol. Embryol. 19: 79-132. Silva, E.C.M. (1999). Caracterização histoquímica das glândulas salivares de Apis mellifera (Hymenoptera, Apidae) durante o desenvolvimento larval. Master’s thesis, Universidade Estadual Paulista, Rio Claro, SP, Brasil. Silva de Moraes, R.L.M. and Cruz-Landim, C. (1975). Ultraestrutura da glândula salivar larval de Apis mellifera adansonii (Hymenoptera, Apidae). Anais do 3º Congresso Brasileiro de Apicultura, Piracicaba, SP, Brazil, pp. 142-152. Silva de Moraes, R.L.M. and Cruz-Landim, C. (1979). Estudos ultra-estruturais da glândula salivar larval de Melipona quadrifasciata anthidioides Lep. durante o desenvolvimento larval. Rev. Bras. Biol. 39: 103-116. Silva-Zacarin, E.C.M., Silva de Moraes, R.L.M. and Taboga, S.R. (2003). Silk formation mechanisms in the larval salivary glands of Apis mellifera (Hymenoptera: Apidae). J. Biosc. 28: 101-112. Velthuis, H.H.W. (1997). Biologia das Abelhas sem Ferrão. ICB-USP, São Paulo, SP, Brazil. Wheeler, G.C. and Wheeler, J. (1976). Ant larvae: review and synthesis. Mem. Entomol. Soc. Wash. 7: 1-108. Wheeler, G.C. and Wheeler, J. (1979). Larvae of social Hymenoptera. In: Social Insects (Hermann, H.R., ed.). Academic Press, London, England. Zara, F.J. (1998). Caracterização morfo-histológica, histoquímica e ultra-estrutural da porção secretora da glândula salivar do quarto estágio larval de Neoponera villosa (Formicidae: Ponerinae). Master’s thesis, UNESP, Rio Claro, SP, Brazil. Zara, F.J. (2002). Estudo químico, bioquímico e citoquímico da região posterior da glândula salivar de larvas do último estágio de formigas Pachycondyla (=Neoponera) villosa (Hymenoptera: Formicidae). Doctoral thesis, UNESP, Rio Claro, SP, Brazil. Zara, F.J. and Caetano, F.H. (1999). Ultrastructure and cytochemistry of fat body in fourth instar of Pachycondyla (=Neoponera) villosa larvae (Formicidae: Ponerinae). V Interamerican Electron Microscopy Congress, October 24-28, 1999, Isla Margarita, Venezuela. CD-Rom. CIASEM, Isla Margarita, Venezuela. Zara, F.J. and Caetano, F.H. (2002). Ultrastructure of the salivary glands of Pachycondyla (=Neoponera) villosa (Fabricius) (Formicidae: Ponerinae): functional changes during the last larval instar. Cytologia 67: 267-280. Zara, F.J. and Caetano, F.H. (2003). Ultramorphology and histology of the larval salivary gland of Pachycondyla villosa (Fabricius) (Hymenoptera: Formicidae, Ponerinae). Neotrop. Entomol. 32: 59-68. Zucchi, R. (1993). Ritualized dominance, evolution of queen-worker interations and related aspects in stingless bees (Hymenoptera: Apidae). In: Evolution of Insect Societies (Inoue, T. and Yamane S., eds.). Hakuhin-Sha Publishing Co., Tokyo, Japan, pp. 207-249. |
|