ABSTRACT. Most research on hygienic behavior has recorded the time taken by the colony to remove an experimental amount of dead brood, usually after one or two days. We evaluated the time that hygienic (H) and non-hygienic (NH) honey bees take to uncap and remove dead brood in observation hives after the brood was killed using the pin-killing assay. Four experimental colonies were selected as the extreme cases among 108 original colonies. Thirty brood cells were perforated with a pin in two H and two NH colonies and observations were made after 1, 2, 3, 4, 5, 6, and 24 h. Different stages of uncapping and removing were recorded. Differences in uncapping and removal between H and NH colonies were significant for all comparisons made at the different times after perforation. Using observation hives one obtains a better and faster discrimination between H and NH colonies than in full size colonies. It is possible to differentiate H and NH within a few hours after perforating the cells. Key words: Honey bees, Hygienic behavior, Uncapping, Removing, Apis mellifera INTRODUCTION Some honey bees (Apis mellifera) perform a behavior that consists of uncapping cells containing dead, sick or damaged brood and removing this brood from the colony. Rothenbuhler (1964) termed this behavior hygienic behavior, and he was the first to study the genetics of this trait. He determined that hygienic behavior is regulated by two pairs of recessive genes; the uncapping gene ”u” (which controls the opening of brood cells) and the removing gene “r” (which controls the removal of the affected brood). Moritz (1988) suggested that the genetic determination of the hygienic behavior probably is more complex, because removing brood from the cells would be controlled by more than two loci, perhaps three. Gramacho (1999) presented a new hypothesis in which the control of this behavior could be explained by three recessive genes (d1/d1, d2/d2 = uncapping and r/r = removal). However, neither hypothesis has been tested. Recently, Lapidge et al. (2002), using molecular techniques, have suggested that seven loci are involved in hygienic behavior. Many authors have demonstrated that hygienic behavior is a natural mechanism of resistance to American foulbrood (Rothenbuhler, 1964; Taber, 1982; Spivak and Reuter, 2001) and chalkbrood diseases (Milne Jr., 1983; Gilliam et al., 1989; Spivak and Gilliam, 1993). Some breeding programs have selected for this trait to increase the frequency of the behavior in honey bee populations (Rothenbuhler, 1964; Taber, 1982; Spivak and Gilliam, 1993; Palacio et al., 2000). Most research on hygienic behavior has recorded the percentage of dead brood uncapped and removed by bees after a certain time. Assays for this behavior are often tested in full-size colonies and results are evaluated after one or two days. Two main methods have been used to elicit hygienic behavior: brood killed by freezing (Gonçalves and Kerr, 1970; Spivak and Reuter, 1998) and brood killed by a pin or “pin-kill test” (Newton and Ostasiewski, 1986; Gonçalves and Gramacho, 1999; Palacio et al., 2000). Both methods have been tested by Gramacho (1995) and Palacio et al. (1996) and have been considered efficient to test this behavior. However, the pin-kill test is both practical and cheaper. Gramacho (1999) studied the different stages of hygienic behavior in full-size colonies at intervals of 2 h after killing the brood by the pin-kill test over 48 h, and recorded the following variables: number of capped cells, empty cells, punctured cells, uncapped cells, and cells with partially removed brood. She suggested that cell uncapping was preceded by the bees poking a hole through the cell capping, and found that hygienic (H) and non-hygienic (NH) colonies performed the stages of uncapping and removing at different rates. It became apparent that shorter intervals can provide more discriminatory evaluations of hygienic behavior than the normal evaluations made after 24 or 48 h. Nevertheless, opening the colonies every 2 h can disturb the bees, and these manipulations may affect the results of the evaluations. Consequently, we evaluated the time that H and NH honeybees take to uncap and to remove dead brood in observation hives after the pin-killing test. MATERIAL AND METHODS One hundred and eight colonies were evaluated for their total hygienic behavior (THB) (Palacio et al., 2000) using the pin-killed test (Newton and Ostasiewsky, 1986, modified by Palacio et al., 2000). All the capped cells present in an area of 10 cm x 5 cm were counted (x). Later, capped brood cells were perforated using a pin to kill the brood. The treated comb was replaced in the original colony and after 24 h the number of uncapped cells with dead brood inside (z) and the number of cells that remained capped (y) were recorded. THB was calculated as the number of cells of dead brood that were removed by the honey bees divided by the total number of cells of brood that had been killed
In order to select the H and NH colonies for this experiment, 108 colonies were tested three times at 15-day intervals and the four colonies with the most extreme values were designated: H colonies (H1 and H2) and NH colonies (NH1 and NH2). The H colonies had an average THB score of 100% and the NH colonies had an average score of 40%. A standard deep, Langstroth size comb containing sealed worker brood covered by honey bees was removed from each colony and was placed inside an observation hive. A new queen was introduced in each hive and observations began when the queen was free and the colony appeared normal. Modified observation hives were used to avoid disturbing the honey bees while perforating the brood. Sixty-five holes (35 mm in diameter) were made in one of the walls of the observation hive and they were covered by small plastic sheets. The plastic sheets were fixed by two sides to the colony wall and they were used as little windows. In this way, it was possible to reach the brood through these windows and handle the colony with minimum disturbance (Figures 1 and 2).
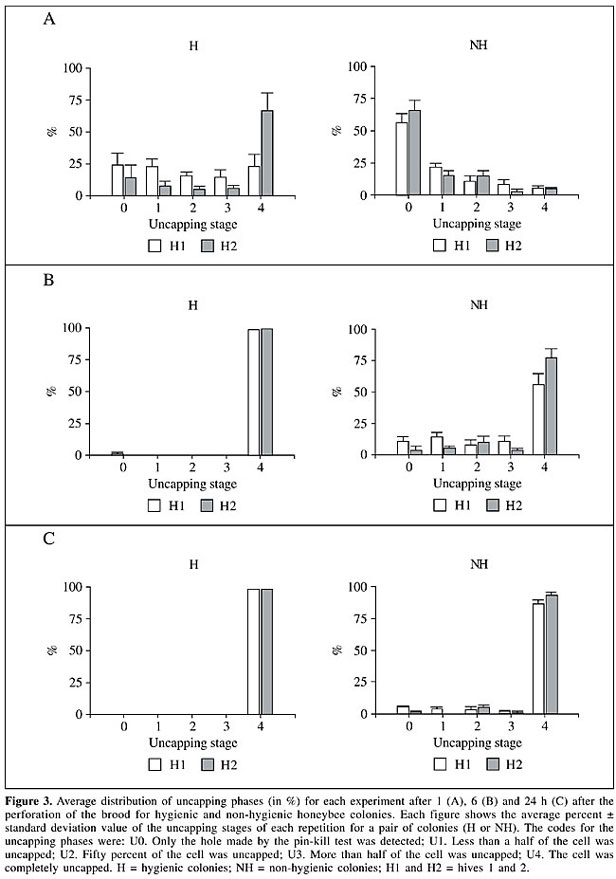
Thirty worker brood cells in each observation hive were perforated using a number 1 insect pin. Observations were done 1, 2, 3, 4, 5, 6, and 24 h after perforating them. The following codes for the stages of uncapping were used: U0. Only the hole made by the pin-kill test was detected. No honey bee activity was detected. U1. Less than a half of the cell was uncapped. U2. Fifty percent of the cell was uncapped. U3. More than half of the cell was uncapped. U4. The cell was completely uncapped. The stages of removal were as follows: R0. The worker brood was untouched. R1. The honey bees began removal but the worker brood was still inside the cell. R2. Less than 50% of the worker brood was removed. R3. Fifty percent of the worker brood was removed. R4. More than 50% of the worker brood was removed. R5. Less than 50% of the worker brood remained in the bottom of the cell. R6. Worker brood was totally removed. These stages of uncapping and removal were recorded in all four colonies on four different days (repetitions). The frequencies of cells with different uncapping and removal stages at each hour and for all colonies were compared using the chi-square test. A two-sided k-sample Smirnov test was performed to compare the time taken by H and NH colonies to uncap and remove the affected brood after 24 h. RESULTS Uncapping stages The percentages of brood cells in different stages of uncapping within H and NH colonies at each observation time were recorded (Figure 3). Differences between H and NH colonies were significant for all comparisons of removal per interval of time after perforation (P < 0.0001). There were also significant differences between repetitions done on different days, but the tendency was the same for each experiment. One hour after the perforation of the worker brood in the H colonies, about 45% (average of the four experiments) of the brood was completely uncapped and 19% (average of the four repetitions) was undisturbed (Figure 3A). After 1 h, a mean of about 45% of the cells in the H colonies were completely uncapped and 19% of the perforated were still undisturbed by the bees (Figure 3A). However, in NH colonies only about 4% of the brood was completely uncapped and 60% was still undisturbed after 1 h. This tendency was seen over all observations (Figure 3). All of the brood of the H colonies was totally uncapped 6 h after perforation (Figure 3B), compared to only 70% in the NH colonies. About 10% of the brood was still capped in the NH colonies 24 h after the perforation of the brood cells (Figure 3C).
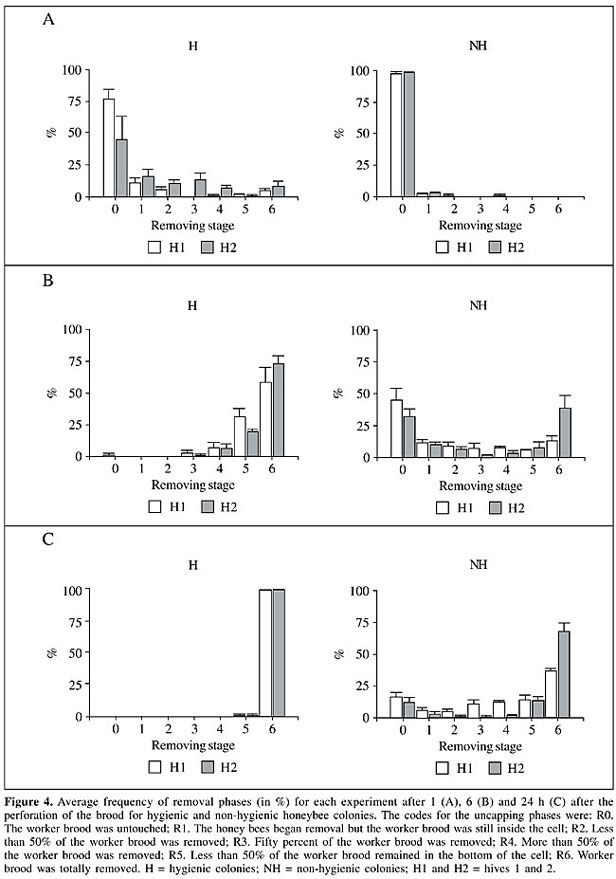
Removal stages The brood removal stage categories evaluated in H and NH were also evaluated at each time interval (Figure 4). Differences in brood removal between H and NH colonies were significant (P < 0.0001) for all observation periods (Figure 4). Significant differences between the repetitions were also detected (data not shown) but the tendency was similar within each pair of colonies. One hour after the perforation of the brood a mean of about 6% of the perforated brood was totally removed in the pair of H colonies (Figure 4A), and no brood was removed in the NH group of colonies. Six hours after perforation the tendency was maintained (Figure 4B), and after 24 h, 99% of the brood had been totally removed in H colonies and about 53% in NH colonies (Figure 4C).
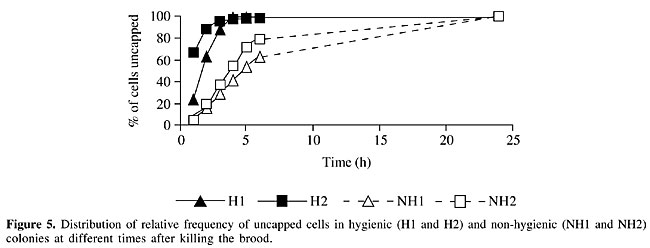
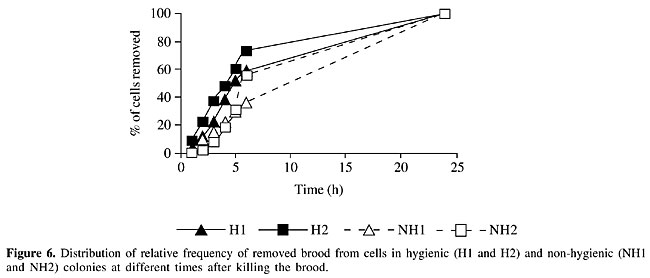
The rate of uncapping and removing brood observed for the 24 h after perforation of the brood was determined in the pairs of H and NH colonies (Figures 5 and 6). In these analyses the number of cells that H or NH colonies effectively uncap or remove was considered as 100% of cells and the relative percentages were calculated. As the NH colonies effectively removed 53% of dead brood (Figure 4), this was corrected to 100% (Figure 6) and the time taken to reach this level was determined.
When uncapping and removal were considered together, the distribution frequency was similar for both colonies within groups (H and NH) but differed between groups. Six hours after perforation, all the H colonies had uncapped all the cells (Figure 5) and about 70% of the brood had already been removed (Figure 6). At this time the NH colonies had uncapped about 70% of the cells (Figure 5) and removed 30% of the brood (Figure 6). This indicates that NH colonies not only uncapped and removed less perforated brood cells than did H colonies, but they took more time to do the job. Differences between distribution frequencies of brood removal were also found to be significant during the first 6 h. DISCUSSION Hygienic colonies uncapped and removed a significantly higher percentage of pin-killed brood than did NH colonies in observation hives. This difference was detected 1 h after the pin-kill test and it was significant for all the time periods. In addition, the time spent by NH colonies to uncap and remove the dead and damaged brood was longer than in H colonies. It is possible to discriminate between colonies that show extremes in hygienic and non-hygienic behaviors within 1 h using observation hives. The mean percentage of totally removed dead or damaged brood 6 h after perforation of the brood by H colonies was 64%, while in NH colonies it was only 26%. Gramacho (1999) recorded values between 1 and 31.6% for removed dead brood 6 h in four different colonies. We found higher rates of removal for both H and NH colonies. When we consider the data obtained after 24 h, NH colonies uncapped 90% of the dead or damaged brood but they only removed 53%. At this time H colonies had uncapped 100% of dead brood and removed 99% of them. Though all differences (uncapped and removed cells) were significant between H and NH colonies for each hour, differences were more obvious when the rate of removal was considered. Palacio et al. (2000) also registered a significant increase in total hygienic behavior (which involves uncapping and removal of dead brood) but not in partial hygienic behavior (which involves uncapping and partial removal of dead brood) in a population selected for this behavior. Gramacho (1999) also found that after 2 h all the colonies tested had some cells uncapped and that maximum values for this variable were detected between 4 and 6 h after killing the brood. Many authors have studied the relation between the expression of hygienic behavior and colony strength. Jones and Rothenbuhler (1964) observed that workers take the same time to remove 2000 or 100 dead larvae from the colony and suggested that honey bees patrolling inside the hive perform cleaning jobs whenever necessary. Spivak and Gilliam (1993) evaluated hygienic behavior in Langstroth hives and in observation hives (containing two frames covered by honey bees). No significant differences were detected in percentages of removed brood in Langstroth hives and observation hives when NH colonies were evaluated, but H colonies removed less brood when they were in observation hives. These authors suggested that hygienic behavior expression was affected by colony strength. We used observation hives with one frame and H colonies removed 99% of dead brood after 24 h while NH colonies had low removal values (53%). Up to now no conclusive evidence exists about the stimuli that release hygienic behavior. Gramacho et al. (1997) indicated that differences in temperature registered between dead and live brood are recognized by workers. Masterman et al. (1998, 2000) detected differences in olfactory discrimination between H and NH honey bees using the proboscis extension reflex. Gramacho et al. (2003) postulated that sensilla placodea have an important role in enabling worker bees to sense sick brood. They studied the number of these structures in H and NH honey bees, but did not find significant differences. We conclude that honey bees are able to detect affected brood during the first hour after killing with the pin-kill test. It is possible that when brood cell is experimentally perforated dead brood odor is more easily detected by hygienic bees, than dead or dying brood with unperforated cappings. We agree with Gramacho (1999) who concluded that it is not necessary to wait 48 h to test the hygienic behavior of a colony because 24 h after the pin-kill test all the punctured brood are already removed. When the pin-killing test is used in observation hives it is possible to evaluate hygienic behavior within a few hours after perforation. ACKNOWLEDGMENTS Research supported by the Argentine National Agency of Scientific and Technological Promotion and International Foundation for Science (Grant No. 2756/1). We thank Claudia Forte for her collaboration. REFERENCES Gilliam, M., Taber, S., Lorenz, B.G.J. and Prest, D.B. (1989). Hygienic honey bees and antagonistic normal microflora for control of chalkbrood disease. XXXII International Apiculture Congress of Apimondia, October 22-28, Rio de Janeiro, RJ, Brazil. Gonçalves, L.S. and Kerr, W.E. (1970). Genética, Seleção e Melhoramento. 1. Noções sobre genética e melhoramento em abelhas. In: Anais do I Congresso Brasileiro de Apicultura, Florianópolis, SC, Brazil, pp. 8-36. Gonçalves, L.S. and Gramacho, K.P. (1999). Seleção de abelhas para resistência a doenças de crias através do comportamento higiênico. Mensagem Doce 52: 2-7. Gramacho, K.P. (1995). Estudo do comportamento higiênico em Apis mellifera, como subsídio a programas de seleção e melhoramento genético em abelhas. M.Sc. thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, USP, Ribeirão Preto, SP, Brazil. Gramacho, K.P. (1999). Fatores que interferem no comportamento higiênico das abelhas Apis mellifera. Ph.D. thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, USP, Ribeirão Preto, SP, Brazil. Gramacho, K.P., Goncalves, L.S. and Rosenkranz, P. (1997). Temperature measurement of living and killed (pin test) honey bee brood (Apis mellifera L). German Bee Research Institutes Seminar. Report of the 44th Meeting in Jena, March, 18-20, 1997. Apidologie 28: 205-207. Gramacho, K.P., Goncalves, L.S., Stort, A.C. and Noronha, A.B. (2003). Is the number of antennal organs (sensilla placodea) greater in hygienic than in non-hygienic Africanized honey bees? Genet. Mol. Res. 2: 309-316. Jones, R.L. and Rothenbuhler, W.C. (1964). Behaviour genetics of nest cleaning in honey bees. II. Response of two inbred lines to various amounts of cyanide killed brood. Anim. Behav. 12: 584-588. Lapidge, K.L., Oldroyd, B.P. and Spivak, M. (2002). Seven suggestive quantitative loci influence hygienic behavior of honey bees. Naturwissenschaften 89: 565-568. Masterman, R., Mesce, K., Smith, B.H. and Spivak, M. (1998). Odor discrimination by hygienic honeybees using proboscis extension conditioning. Am. Bee J. 138: 297-298. Masterman, R., Smith, B.H. and Spivak, M. (2000). Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J. Insect Behav. 13: 87-101. Milne Jr., C.P. (1983). Honey bee (Hymenoptera: Apidae) hygienic behaviour and resistance to chalkbrood. Ann. Entom. Soc. Am. 76: 384-387. Moritz, R.F.A. (1988). A re-evaluation of the two locus model for hygienic behaviour in honeybees (Apis mellifera L.). J. Hered. 79: 257-262. Newton, D.C. and Ostasiewski , N.J.A. (1986). A simplified bioassay for behavioral resistance to American Foulbrood in honey bees (Apis mellifera L). Am. Bee J. 126: 278-281. Palacio, M.A., Figini, E., Rodriguez, E., Del Hoyo, M., Ruffinengo, S. and Bedascarrasbure, E. (1996). Relación entre el comportamiento higiénico en Apis mellifera y la fortaleza de la colonia”. In: V Congreso Iberolatinoamericano de Apicultura, Mercedes, Uruguay, 153. Palacio, M.A., Figini, E., Ruffinengo, S., Rodriguez, E., Del Hoyo, M. and Bedascarrasbure, E.L. (2000). Changes in a population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie 31: 471-478. Rothenbuhler, W.C. (1964). Behavior genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood. Am. Zool. 4: 111-123. Spivak, M. and Gilliam, M. (1993). Facultative expression of hygienic behaviour of honey bees in relation to disease resistance. J. Apic. Res. 32: 147-157. Spivak, M. and Reuter, G.S. (1998). Honey bee hygienic behavior. Am. Bee J. 138: 283-286. Spivak, M. and Reuter, G.S. (2001). Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32: 555-565. Taber, S. (1982). Bee behavior: Breeding for disease resistance. Am. Bee J. 122: 823-825. |
|