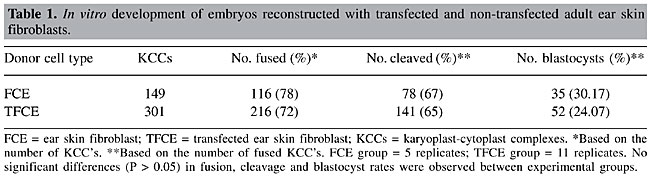
ABSTRACT. An association of two techniques, nuclear transfer (NT), and transfection of somatic animal cells, has numerous potential applications and considerable impact, mainly in agriculture, medicine, pharmacy, and fundamental biology. In addition, somatic cell nuclear transfer is the most efficient alternative to produce large transgenic animals. We compared in vitro and in vivo developmental capacities of NT using fibroblast cells isolated from a 14-month-old cloned Simmental heifer (FCE) vs the same line transfected with a plasmid containing neomycin-resistant genes (TFCE). There were no significant differences (P > 0.5) in either fusion (116/149 = 78% vs 216/301 = 72%), cleavage (78/116 = 67% vs 141/216 = 65%) and blastocyst (35/116 = 30% vs 52/216 = 24%) rates or in pregnancy rate at 30 to 35 days after embryo transfer (2/17 vs 3/17) between NT using FCE and TFCE, respectively. Transfection and long-term in vitro culture of transfected cells did not affect developmental capacity of NT embryos up to 40 days of gestation. Key words: Bovine, Assisted reproduction technology, Transfection, Transgenic animals, Fibroblasts INTRODUCTION The potential applications of somatic cell nuclear transfer (SCNT) technique have received much attention since the first births of cloned animals were reported in various domestic species, including sheep (Wilmut et al., 1997), cattle (Kato et al., 1998), goat (Baguisi et al., 1999), pig (Onishi et al., 2000; Polejaeva et al., 2000), and more recently, horse (Galli et al., 2003). Consequent benefits affect many areas, including basic biological studies, agricultural livestock improvement and animal conservation. However, the greatest impact of SCNT, due to its association with the genetic manipulation field, has been the possibility to add (“knock-in”) or to inactivate/suppress (“knock-out”) genes of interest in domestic species (see Hodges and Stice, 2003). Transgenic animals have numerous attractive applications in biomedicine (such as production of recombinant human proteins, nutraceutical production, xenotransplantation, and human genetic disease models) as well as for improving livestock production traits (disease and pest resistance, more efficient feed conversion, muscle composition, and milk composition for manufacturers). Considering the aforementioned reasons, many laboratories and companies are focusing on understanding and surmounting low efficiency and developmental abnormalities associated with nuclear transfer (NT) in hopes of overcoming impediments to the ultimate benefits of transgenic livestock clones (Campbell et al., 2001) and commercial exploitation of recombinant proteins produced by animal bioreactors (Keefer, 2004). Many cellular and molecular mechanisms involved in these impediments must still be studied. The information provided by complete genome sequences of domestic animals will improve gene-targeting technology (Niemann and Kues, 2003). Increasing NT efficiency, through adult somatic donor cells instead of fetal or embryonic cells, is very important for the following reasons. First, a well-known and characterized phenotype and genotype are recommended for animal clone candidates, especially those in a transgenic program. Second, not only are somatic cells relatively easy to isolate, culture and replicate in vitro, they are also an abundant source of donor nuclei. Transfection or gene targeting of NT donor cells both require a long in vitro culture period and selection of marker genes. Few studies (Zakhartchenko et al., 2001; Arat et al., 2001; Bhuiyan et al., 2004) have evaluated the effects of long-term culture and genetic manipulation of adult somatic donor cells for NT in bovines. Moreover, related reports (Brink et al., 2000; Zakhartchenko et al., 2001; Arat et al., 2001, 2002) have presented conflicting results concerning the developmental capacity of embryos reconstructed with transfected versus non-transfected donor cells. Consequently, we evaluated in vitro and in vivo developmental capacities of NT using fibroblast cells isolated from the ear skin of a cloned Simmental heifer (FCE) and the same cell line transfected (lipofection) with an expression vector containing neomycin-resistant genes under the control of the SV40 promoter (TFCE). MATERIAL AND METHODS Unless stated otherwise, all chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA). Isolation and culture of ear skin fibroblasts Fibroblasts were isolated from a small ear skin biopsy of a 14-month-old NT Simmental heifer (Sousa et al., 2000; Iguma et al., 2001) as follows: the biopsy was dissected after four baths in modified Dulbecco’s phosphate-buffered saline (mDPBS) to separate the internal cartilage, which was then minced into smaller pieces and distributed on the bottom of 25-cm2 flasks (Corning, New York, NY, USA) filled with 2 ml Dulbecco’s modified Eagle’s medium (D-MEM; Invitrogen, Carlsbarg, CA, USA) supplemented with 10% fetal calf serum (FCS; Invitrogen) and penicillin/streptomycin (100 IU/ml penicillin G and 50 µg/ml streptomycin). The flasks were then placed in an incubator at 39°C with 5% CO2 in air with saturated humidity. Once the fibroblasts reached complete confluence, cells were trypsinized and either frozen for storage (in liquid nitrogen), cultured in vitro or prepared for later transfection. The freezing protocol utilized the above-mentioned fibroblast culture medium with an added 10% dimethyl sulfoxide (DMSO). Fibroblast cells were kept in 100% confluence for four to five days prior to preparation for NT manipulation. The cell monolayer was washed twice with mDPBS before incubation in 0.5 ml 0.25% trypsin and 0.1% EDTA diluted in Hanks’ salt solution (Invitrogen) for 10 min at 39°C. Next, 3 ml D-MEM with 10% FCS was added to neutralize trypsin activity. For cell recovery, 3.5 ml content was centrifuged at 200 g for 5 min, and the supernatant was discarded. Centrifuge parameters were repeated twice for washing fibroblast cells in HEPES buffer TCM 199 (Invitrogen) supplemented with 10% FCS, 100 IU/ml penicillin and 50 µg/ml streptomycin (manipulation medium) - the same medium in which donor cells were resuspended (200 µl) and kept until the embryo reconstruction process. Stable fibroblast transfection Plasmid vector pCI-neo (Promega, Madison, WI, USA) was used for stable fibroblast cell transfection. This vector carries a poly-A sequence and the neomycin gene under control of the CMV promoter sequence. The plasmid was amplified in a DH5a strain of Escherichia coli and purified using the Qiagen Plasmid Giga Kit (Qiagen, Valencia, CA, USA). Bovine fibroblast cells were plated at 2 x 105 into 24-well culture dishes and transfected with 0.5 µg pCI-neo and Lipofectamine (8.3 µl/ml - Invitrogen), according to the manufacturer’s guidelines. Forty-eight hours after transfection, cells were diluted to 1:10, and G418 (neomycin) was added at a final concentration of 0.4 mg/ml. Transfected cells achieved 70 to 80% of confluence after seven days of selection. At the third passage, one portion of cells was diluted 1:10, cultured under antibiotic selective pressure for seven days and utilized for NT. Another portion of the cells was cryopreserved as a mixed cell population and expanded for isolation of DNA and use in polymerase chain reaction (PCR) and Southern blot analyses. Polymerase chain reaction analysis Genomic DNA of bovine transfected fibroblasts was extracted and purified with a Wizard® Genomic DNA Purification Kit (Promega). Genomic DNA was used as a template in PCR reactions containing specific neo gene primers (410 bp): NPT60 (5’-GAGGCTATTCGGCTATGACTG-3’) and NPT470c (5’-TCGACAAGACCGGCTTCCATC-3’). PCR reactions were carried out in a final volume of 25 µl, containing 10 mM Tris-HCl, pH 8.4, 50 mM KCl, 2 mM MgCl2, 160 µM of each dNTP, 0.4 µM of each primer (NPT60 and NPT470c), and 2 U Taq polymerase (Invitrogen). The PCR reaction consisted of 35 cycles (94°C/2 min, 94°C/30 s, 59°C/30 s, 68°C /1 min, and 68°C/7 min) in a PT-100 thermal cycler (MJ Research, San Francisco, CA, USA). Amplified PCR products were analyzed in 1.0% agarose gel stained with ethidium bromide (50 ng/ml), and images were digitalized using Eagle Eye (Stratagene, La Jolla, CA, USA). Oocyte recovery and in vitro maturation Ovaries collected from a local slaughterhouse, which had 2- to 8-mm follicles, were punctured with a 19-gauge scalp connected to a vacuum system. Cumulus-oocyte complexes (COCs) were recovered and classified based on granulosa cell layers and cytoplasm homogeneity (classes 1 to 4). Twenty to 25 grade 1 and 2 COCs were matured in vitro in 400 µl drops of TCM 199 (Invitrogen) supplemented with 10% FCS (Invitrogen), 24 IU/ml LH, 10 µg/ml FSH, 100 IU/ml penicillin and 50 µg/ml streptomycin, covered with mineral oil and kept at 39°C in a 5% CO2 air atmosphere. Nuclear transfer and artificial activation Between 19 to 22 h after in vitro maturation (IVM) onset, COCs were denuded by pipetting in 0.2% (w:v) hyaluronidase. Oocytes were separated according to the first polar body extrusion and cytoplasm quality. Only mature, good quality oocytes were micromanipulated and used for enucleation. Prior to enucleation, oocytes were incubated for 15 min in 2 µg/ml Hoechst 33342 and 3.33 µg/ml cytochalasin D diluted in IVM medium. Two cell types were tested for reconstruction: non-transfected and transfected adult fibroblasts. The donor cell was then placed within the perivitelline space, adjacent to the plasma membrane of the oocyte. Next, the karyoplast-cytoplasm complexes (KCCs) were placed between two electrodes overlaid with fusion medium (0.28 M mannitol, 1 mM CaCl2, 0.1 mM MgCl2) and manually aligned before applying two DC pulses of 2.1 kV/cm each for 30 µs, delivered by a BTX Electrocell Manipulator 200 (BTX, San Diego, CA, USA) (day 0 - D0). Fusion was confirmed under a stereomicroscope (Stemi SV11, Zeiss, Oberkochen, Germany) 1 h after electrical stimuli, and KCCs recorded as non-fused were submitted to another round of electrofusion. The KCCs were artificially activated 3-5 h after fusion using 5 µM ionomycin for 5 min, followed by incubation for 5 h in 2 mM 6-dimethylaminopurine (6-DMAP) prepared in SOFaaci (Holm et al., 1999) with the addition of 5% FCS. A sample of matured oocytes from each manipulation was separated for parthenogenetic activation (activation control group), following the protocol of reconstructed embryos activation. Afterwards, the KCCs and the activation control group were cultured in vitro. Embryo culture, embryo transfer and pregnancy assessment Presumptive NT and parthenogenetic embryos were co-cultured with a granulosa cell monolayer in 200 µl drops (15 to 25 structures) of SOFaaci supplemented with 5% FCS covered with mineral oil at 39°C and 5% CO2 in humidified air. Development rates were recorded on day 2 (D2) for cleavage and D6 to D7 for blastocyst rate. One to three blastocysts derived from FCE and TFCE were placed into a 0.25-ml straw (IMV, L’Aigle, France) filled with TQC Holding Plus (AB Technology, Pullman, WA, USA) for transportation until non-surgical transfer to synchronous recipients. These cows were monitored every 30 days after embryo transfer by ultrasound scanning and/or palpation via the rectum for pregnancy, fetal and placental development. Fetal recovery and fibroblast isolation Two fetuses at 44 days of gestation were surgically recovered from the uterus of a single recipient (double-embryo transfer) from the TFCE experimental group for molecular analysis. Primary fetal fibroblast cells were prepared as follows: fetuses were washed in mDPBS before removing heads and soft tissues with tweezers and scissors. Carcasses were minced, and fetal skin tissues were cultured in a manner similar to the ear skin fibroblast. After three passages, cells were frozen using 10% DMSO in D-MEM supplemented with 10% FCS, and were subsequently stored in liquid nitrogen. Southern blot analysis Genomic DNA (20 mg) from transfected fetal (N = 2) fibroblasts and non-transfected adult ear (N = 1) fibroblast cells was isolated and purified using a Wizard® Genomic DNA Purification Kit (Promega). The DNA was then digested with BamHI (Promega), and the fragments were separated by electrophoresis in 1.0% agarose gel and transferred to a N+ nylon membrane (Amersham). A 719-bp probe (50 ng) from within the neo gene-coding sequence was prepared by digesting the pCI-neo vector with NcoI. Probes were labeled with a32P dCTP (3000 Ci/mol) using a random primer DNA labeling kit (Pharmacia Biotech) according to the manufacturer’s instructions. Hybridization was carried out following standard procedures (Sambrook et al., 1989). Statistical analysis The effects of transfection on the in vitro developmental capacity of embryos reconstructed with either transfected or non-transfected adult fibroblast donor cells were evaluated using a chi-square test (SigmaStat 2.0; Jandel Scientific, San Rafael, CA, USA). The Fisher exact test (SigmaStat 2.0) was applied to analyze in vivo development of the two groups. P < 0.05 was considered to be significantly different. RESULTS The average rates of parthenogenetically activated oocytes for the control of activation procedures and culture system (330 oocytes in 7 replicates) were 69.4% for cleavage and 40.3% for blastocyst rates. In vitro developmental capacity of nuclear transfer embryos NT efficiency in transfected and non-transfected adult ear skin fibroblasts was compared (Table 1). No significant differences in terms of fusion, cleavage and blastocyst rates were found between embryos derived from transfected versus non-transfected fibroblast cells.
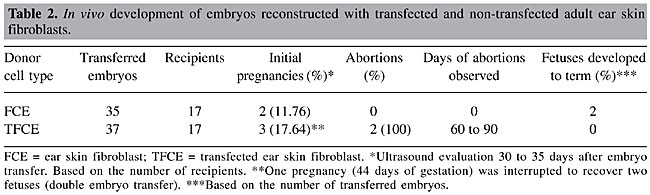
In vivo developmental capacity of nuclear transfer embryos All FCE embryos reaching blastocyst stage (N = 35) were transferred to 17 recipients. Within the TFCE group, some blastocysts were separated for PCR analysis (data not shown), and 37 of 52 embryos were transferred to 17 recipients (Table 2). Ultrasound evaluation detected no significant differences in initial pregnancy rate between transfected and non-transfected fibroblast groups. Two embryos from FCE group and no embryos from TFCE group developed to term. Both calves (66 and 62 kg) were heavier than average for females of this breed (39 to 42 kg). Moreover, clones had longer gestation periods (305 and 294 days) than the average expected for the Simmental breed (282 days). The heaviest clone died as a consequence of dystocia at parturition, while the other was delivered by cesarean section and survived for four months. Only the former pregnancy displayed a moderate degree of hydroallantois. Parentage of the cloned calves was confirmed by comparing 11 microsatellite markers from donor and cloned cell lines with those of the recipients (data not shown).
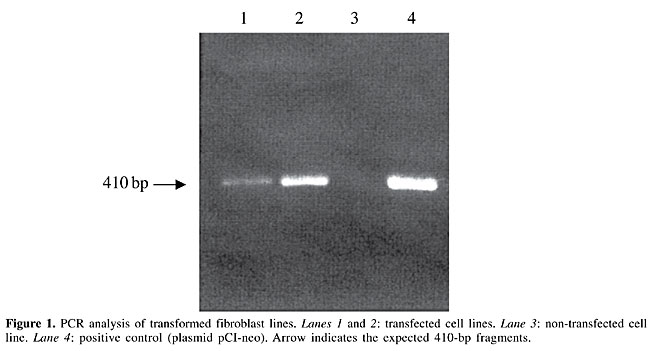
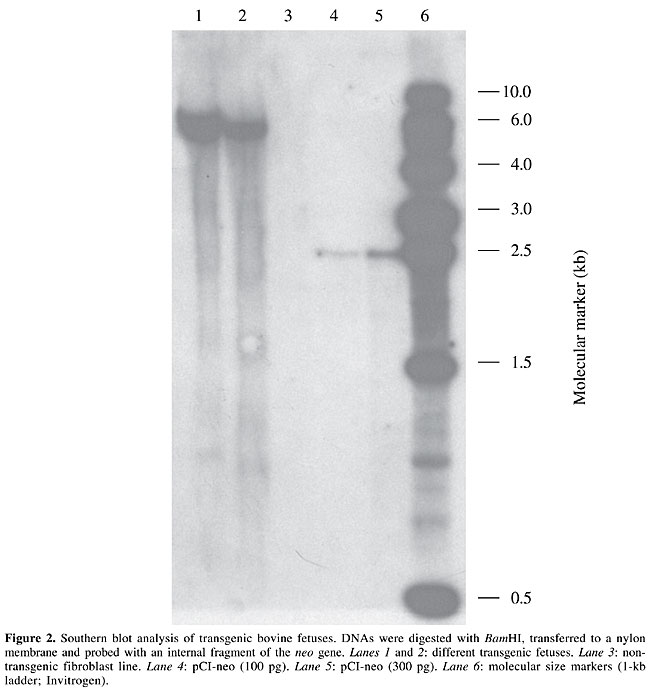
Molecular analyses PCR analysis confirmed presence of the neo gene in fibroblast lines growing on G-418 (Figure 1). One of these lines was utilized in NT procedures in order to generate genetically modified embryos. Southern blot analysis of genomic DNA isolated from the fibroblasts of the two fetuses was carried out to evaluate integration of the introduced neo gene. It was found in both fetuses. DNA isolated from a non-transgenic fibroblast line did not hybridize the neo probe (Figure 2).
DISCUSSION We conclude that somatic nuclear donor cell transfection does not negatively influence in vitro efficiency rates (percentage of fusion, cleavage and blastocyst) of the NT process, as was also concluded by Roh et al. (2000) and Han et al. (2001) for bovines. Likewise, published data from enhanced green fluorescent protein (EGFP) gene-transfected porcine fetal fibroblasts (Koo et al., 2001; Martinez Diaz et al., 2003) corroborate our findings. However, findings have been contradictory in cattle, even in reports from the same research group. Arat et al. (2001) reported similar efficiencies of transfected and non-transfected adult granulosa cells for cleavage and blastocyst rates. Later, Arat et al. (2002) demonstrated that adult and fetal cell lines expressing EGFP had lower in vitro NT developmental capacity than the same non-expressing EGFP lines, whereas transfected but negative expressing-EGFP cells were similar to non-transfected fibroblasts in terms of cleavage and blastocyst rates in NT units. More examples of contradictory results have been observed in bovines; some reports (Zakhartchenko et al., 2001; Bhuiyan et al., 2004) indicated a significant decrease in blastocyst rate when using transfected cells compared to non-transfected donor cells, while others did not report a difference in blastocyst development rate between NT of transfected and non-transfected cells (Brink et al., 2000; Brophy et al., 2003). Zakhartchenko et al. (2001) attributed their decreased blastocyst rate of embryos reconstructed with transfected fetal fibroblasts compared to non-transfected cells to the extended culture period required for the transfection and selection processes, but not to the transgene per se. However, some studies have demonstrated that cells cultured in vitro for a long period (10 to 15 passages; Kubota et al., 2000) had better cleavage and blastocyst rates than cells from earlier passages (<10). The latter data were similar to those recorded by Arat et al. (2001), using transfected and non-transfected adult granulosa donor cells. Even cells near senescence did not exhibit significant differences in in vitro developmental rates compared to earlier passages (Lanza et al., 2000). A recent work (Bhuiyan et al., 2004) in the SCNT bovine field demonstrated that transfected ear fibroblasts were less able to develop to the blastocyst stage than their non-transfected counterpart, regardless of passage number (early or late). More likely, discrepancies among results reported up to now are due to differences in vector type, transfection protocols (Bhuiyan et al., 2004), NT methods and donor cell culture conditions (Wells et al., 2003; Miyoshi et al., 2003). Furthermore, the site of gene(s) integration(s), the transgene used and its possible interference in endogenous gene expression could influence results (Hodges and Stice, 2003). Another aspect to consider is donor cell lines and genotypes, since Heyman et al. (2002) found that four different subcultures isolated from a single skin biopsy resulted in significantly different NT blastocyst rates (ranging from 5 to 30%), and Kühholzer et al. (2001) even found different morula and blastocyst rates among clonal colonies obtained from the same donor cell fetus. In our investigation of in vivo developmental capacity after transfer of NT embryos to recipient cows, we did not observe significant differences in initial pregnancy (< day 40 of gestation) rates between transfected and non-transfected donor cells, which was also reported by Zakhartchenko et al. (2001). In contrast, Forsberg et al. (2002) reported lower rates of initial pregnancy for NT of transfected cells than for NT of non-transfected cells. In our study, the small number of recipients carrying transgenic and non-transgenic cloned embryos precludes a conclusive result concerning embryo survival to term. Nevertheless, we suggest that to some extent transfection procedures negatively affect fetal viability, since non-transgenic pregnancies were miscarried during the first trimester of gestation, though one initial TFCE pregnancy (44 days of gestation) was interrupted. On the other hand, two pregnancies of the FCE group were carried to term. This is supported by the findings of Zakhartchenko et al. (2001) and Brophy et al. (2003) who observed a lower pregnancy rate (> day 30 of gestation) and a lower proportion of viable calves at weaning for NT using transfected cells than for their non-transfected counterpart. Decreased pregnancy rates found in transfected embryos may in part be explained by the greater incidence of apoptosis in SCNT blastocysts derived from long-term (>30 population doublings (PD) for adult ear fibroblasts and >50 PD for fetal fibroblasts) cultured nuclear donor cells than for fresh cell lines (<16 PD for adult and fetal fibroblasts) as suggested by Jang et al. (2004). These authors also demonstrated that in vitro development parameters (blastocyst rate and blastocyst differential cell count) are not affected by extended cell culture periods. The fibroblast line used in the present study was isolated from the ear skin of an embryonic cloned heifer (Sousa et al, 2000; Iguma et al., 2001). This cell line was selected based on its reasonable NT results reached in pre-experiments. Moreover, its assessment using conventional karyotyping and transfection rates showed a low incidence of chromosomal abnormalities (data not shown) and a high frequency of transfection (60-70%; Oliveira et al., 2001). The cytogenetic approach applied to NT donor cells is relevant, since Bureau et al. (2003) reported a direct relationship between chromosomal anomalies in NT embryos and in their respective nuclear donor cells. Striving for cell cycle synchronization, donor cells were maintained in confluence (100%) for four to five days prior to NT instead of serum starvation in fibroblast culture media. For this procedure, we considered the study of Kubota et al. (2000), who found similar rates of cells in G0/G1 phase between serum-starved (83-85%) and confluent cultured cells (82-90%). In addition, Hill et al. (2000) reported similar NT blastocyst rates between serum-starved and serum-fed fibroblasts obtained from a 21-year-old bull, and Cibelli et al. (1998) demonstrated that even the use of fetal fibroblast serum deprivation is not a sine qua non condition to produce viable calves through NT as previously reported (Wilmut et al., 1997). Furthermore, Kues et al. (2002) noticed deleterious effects of serum starvation in culture media of NT donor cells. For cloning purposes, the use of adult somatic cells is more advisable than fetal cells, especially since this option allows selection of animals with increased production traits (high milk yield and growth rate), valuable genetic merit and ensured sanitary safety. These characteristics are not only desirable but are also required for transgenic animal production, due to elevated financial costs and the amount of time involved. To date, there are few studies of the nuclear transfer process using genetically modified adult somatic nuclear donor cells. Since pCI-neo has a unique BamHI restriction site, Southern blot analysis of the transgenic fetuses allowed us to confirm plasmid integration as well as to indicate the presence of a single copy of the neo gene in the bovine genome. Both fetuses presented the same hybridization pattern, since they were generated from the same transfected fibroblast line. Transgene integration at the same locus is a desirable trait for the introduction of transgenic animals in a breeding program directed towards gene product development. In summary, we did not find significant differences in NT developmental rates between transfected and non-transfected adult fibroblast cells. In other words, we observed no deleterious effect of the transfection procedure up to day 40 of pregnancy; however, the low pregnancy rates that we obtained limited comparisons between FCE and TFCE groups concerning gestation to term. Only the former treatment group produced offspring. The TFCE pregnancies were miscarried during the first trimester, except for one that was prematurely interrupted for fetus recovery, to allow transgene analyses. ACKNOWLEDGMENTS Research supported by grants from Embrapa/Cenargen, CNPq and FAP/DF. The authors thank Dr. Adilson Leite (in memoriam) for his efforts to construct the vector. We also thank the Centro Experimental Sucupira staff for care of the animals. REFERENCES Arat, S., Rzucidlo, S.J., Gibbons, J., Miyoshi, K. and Stice, S.L. (2001). Production of transgenic bovine embryos by transfer of transfected granulosa cells into enucleated oocytes. Mol. Reprod. Dev. 60: 20-26. Arat, S., Gibbons, J., Rzucidlo, S.J., Respess, D.S., Tumlin, M. and Stice, S.L. (2002). In vitro development of bovine nuclear transfer embryos from transgenic lines of adult and fetal fibroblast cells of the same genotype. Biol. Reprod. 66: 1768-1774. Baguisi, A., Behboodi, E., Melican, D.T., Pollock, J.S., Destrempes, M.M., Cammuso, C., Williams, J.L., Nims, S.D., Porter, C.A., Midura, P., Palacios, M.J., Ayres, S.L., Denniston, R.S., Hayes, M.L., Ziomek, C.A., Meade, H.M., Godke, R.A., Gavin, W.G., Overström, E.W. and Echelard, Y. (1999). Production of goats by somatic cell nuclear transfer. Nat. Biotech. 17: 456-461. Bhuiyan, M.M.U., Cho, J., Jang, G., Park, E., Kang, S., Lee, B. and Hwang, W. (2004). Effect of transfection and passage number of ear fibroblasts on in vitro development of bovine transgenic nuclear transfer embryos. J. Vet. Med. Sci. 66: 257-261. Brink, M.F., Bishop, M.D. and Pieper, F.R. (2000). Developing efficient strategies for the generation of transgenic cattle which produce biopharmaceuticals in milk. Theriogenology 53: 139-148. Brophy, B., Smolenski, G., Wheeler, T., Wells, D., L’Huillier, P. and Laible, G. (2003). Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat. Biotechnol. 21: 157-162. Bureau, W.S., Bordignon, V., Leveillee, C., Smith, L.C. and King, W.A. (2003). Assessment of chromosomal abnormalities in bovine nuclear transfer embryos and in their donor cell. Clon. Stem Cells 5: 123-132. Campbell, K.H.S., Alberio, R., Lee, J.H. and Ritchie, W.A. (2001). Nuclear transfer in practice. Clon. Stem Cells 3: 201-208. Cibelli, J.B., Stice, S.L., Golueke, P.J., Kane, J.J., Jerry, J., Blackwell, C., Ponce de León, F.A. and Robl, J.M. (1998). Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science 280: 1256-1258. Forsberg, E.J., Strelchenko, N.S., Augenstein, M.L., Betthauser, J.M., Childs, L.A., Eilertsen, K.J., Enos, J.M., Forsythe, T.M., Golueke, P.J., Koppang, R.W., Lange, G., Lesmeister, T.L., Mallon, K.S., Mell, G.D., Misica, P.M., Pace, M.M., Pfister-Genskow, M., Voelker, G.R., Watt, S.R. and Bishop, M.D. (2002). Production of cloned cattle from in vitro systems. Biol. Reprod. 67: 327-333. Galli, C., Lagutina, I., Crotti, G., Colleoni, S., Turini, P., Ponderato, N., Duchi, R. and Lazzari, G. (2003). Pregnancy: a cloned horse born to its dam twin. Nature 424: 635. Han, Y.M., Koo, D.B., Kang, Y.K., Choi, Y.H., Park, J.S., Kim, S.J., Lee, C.S., Kim, T.H. and Lee, K.K. (2001). Production of a transgenic calf by nuclear transfer using transformed cells. Theriogenology 55: 270 (Abstract). Heyman, Y., Zhou, Q., Lebourhis, D., Chavatte-Palmer, P., Renard, J.P. and Vignon, X. (2002). Novel approaches and hurdles to somatic cloning in cattle. Clon. Stem Cells 4: 47-55. Hill, J.R., Quinton, A.W., Long, C.R., Looney, C.R., Thompson, J.A. and Westhusin, M.E. (2000). Developmental rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol. Reprod. 62: 1135-1140. Hodges, C.A. and Stice, S.L. (2003). Generation of bovine transgenics using somatic cell nuclear transfer. Reprod. Biol. Endocrinol. 1: 81. Holm, P., Booth, P.J., Schmidt, M.H., Grave, T. and Callesen, H. (1999). High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol or without serum-proteins. Theriogenology 52: 683-700. Iguma, L., Santos, E.S., Sousa, R.V., Nascimento, N., Câmara, J.U. and Rumpf, R. (2001). Vitória da Embrapa - Primeiro produto brasileiro obtido por transferência nuclear. Dados do parto aos 6 meses de idade. Anais do VI Encontro Talento Estudantil 6: 125. Jang, G., Park, E.S., Cho, J.K., Bhuiyan, M.M.U., Lee, B.C., Kang, S.K. and Hwang, W.S. (2004). Preimplantational embryo development and incidence of blastomere apoptosis in bovine somatic cell nuclear transfer embryos reconstructed with long-term cultured donor cells. Theriogenology 62: 512-521. Kato, Y., Tani, T., Sotomaru, Y., Kurokawa, K., Kato, J., Doguchi, H., Yasae, H. and Tsunoda, Y. (1998). Eight calves cloned from somatic cells of a single adult. Science 282: 2095-2098. Keefer, C.L. (2004). Production of bioproducts through the use of transgenic animal models. Anim. Reprod. Sci. 82-83: 5-12. Koo, D.B., Kang, Y.K., Choi, Y.E., Park, J.S., Kim, H.N., Kim, T., Lee, K.K. and Han, Y.M. (2001). Developmental potential and transgene expression of porcine nuclear transfer embryos using somatic cells. Mol. Reprod. Dev. 58: 15-21. Kubota, C., Yamakuchi, H., Todoroki, J., Mizoshita, K., Tabara, N., Barber, M. and Yang, X. (2000). Six cloned calves produced from adult fibroblast cells after long-term culture. Proc. Natl. Acad. Sci. USA 97: 990-995. Kues, W.A., Carnwath, J.W., Paul, D. and Niemann, H. (2002). Cell cycle synchronization of porcine fetal fibroblasts by serum deprivation initiates a nonconventional form of apoptosis. Clon. Stem Cells 4: 231-243. Kühholzer, B., Hawley, R.J., Lai, L., Kolber-Simonds, D. and Prather, R.S. (2001). Clonal lines of transgenic fibroblast cells derived from the same fetus result in different development when used for nuclear transfer in pigs. Biol. Reprod. 64: 1695-1698. Lanza, R.P., Cibelli, J.B., Blackwell, C., Cristofalo, V.J., Francis, M.K., Baerlocher, G.M., Mak, J., Schertzer, M., Chavez, E.A., Sayer, N., Landsdorp, P.M. and West, M.D. (2000). Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 228: 665-669. Martinez Diaz, M.A., Mori, T., Nagano, M., Katagiri, S. and Takahashi, Y. (2003). Effect of fusion/activation protocol on in vitro development of porcine nuclear transfer embryos constructed with foreign gene-transfected fetal fibroblasts. J. Vet. Med. Sci. 65: 989-994. Miyoshi, K., Rzucidlo, S.J., Pratt, S.L. and Stice, S.L. (2003). Improvements in cloning efficiencies may be possible by increasing uniformity in recipient oocytes and donor cells. Biol. Reprod. 68: 1079-1086. Niemann, H. and Kues, W.A. (2003). Application of transgenesis in livestock for agriculture and biomedicine. Anim. Reprod. Sci. 79: 291-317. Oliveira, R.R., Lisauskas, S., Carvalho, D.M., Vianna, G.R., Dode, M.A.N., Aragão, J.F.L., Rumpf, R. and Rech, E.L. (2001). High-frequency transfection of bovine and ovine fibroblasts. Anais do 47º Congresso Nacional de Genética [CD-ROM]. Sociedade Brasileira de Genética, Ribeirão Preto, SP, Brazil. Onishi, A., Iwamoto, M., Akita, T., Mikawa, S., Takeda, K., Awata, T., Hanada, H. and Perry, A.C. (2000). Pig cloning by microinjection of fetal fibroblast nuclei. Science 289: 1188-1190. Polejaeva, I.A., Chen, S.H., Vaught, T.D., Page, R.L., Mullins, J., Ball, S., Dai, Y., Boone, J., Walker, S., Ayares, D.L., Colman, A. and Campbell, K.H. (2000). Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407: 86-90. Roh, S., Shim, H., Hwong, W. and Yoon, J. (2000). In vitro development of green fluorescent protein (GFP) transgenic bovine embryos after nuclear transfer using different cell cycles and passages of fetal fibroblasts. Reprod. Fertil. Dev. 12: 1-6. Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York, NY, USA. Sousa, R.V., Iguma, L.T., Nascimento, N., Carmo, T.F.M. and Rumpf, R. (2000). Cloning of frozen thawed bovine embryos using pre-activated recipient cytoplasts. Arq. Fac. Vet. UFRGS 28: 335-336. Wells, D.N., Laible, G., Tucker, F.C., Miller, A.L., Oliver, J.E., Xiang, T., Forsyth, J.T., Berg, M.C., Cockrem, K., L’Huillier, P.J., Tervit, H.R. and Oback, B. (2003). Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology 59: 45-59. Wilmut, I., Schnieke, A.E., McWhir, J., Kind, A.J. and Campbell, K.H.S. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature 385: 810-813. Zakhartchenko, V., Mueller, S., Alberio, R., Schernthaner, W., Stojkovic, M., Wenigerkind, H., Wanke, R., Lassnig, C., Mueller, M., Wolf, E. and Brem, G. (2001). Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol. Reprod. Dev. 60: 362-369. |
|