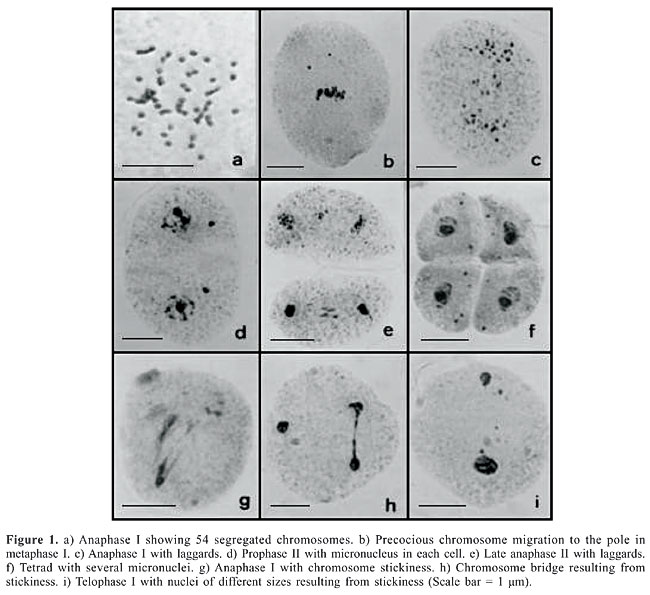
ABSTRACT. In the hexaploid (2n = 6x = 54) accession B176 of Brachiaria brizantha, one cytological characteristic differentiated it from the other accessions previously analyzed with the same ploidy level. Nearly 40% of meiocytes displayed the chromosome set arranged at two metaphase plates at the poles of the cell, close to the membrane. In these cells, both metaphase plates were arranged in an angle to form a typical tripolar spindle. Therefore, cells did not show normal chromosome segregation at anaphase I. Only nine univalent chromosomes migrated from each plate to the opposite pole with the remainder staying immobile on the plate. As a result of such spindle orientation and chromosome behavior, trinucleate telophases I were recorded. After telophase, cytokinesis eliminated the small nuclei into a microcyte. The second division proceeded normally, with the presence of microcytes in all phases. The origin of such an abnormality was explained on the hexaploid level of the accession which could have resulted by chromosome doubling of a triploid derived from species that did not display the same behavior for spindle organization. The high percentage of meiotic abnormalities recorded in this accession compromises fertility and renders it inadequate for the breeding program. Key words: Brachiaria brizantha, Allopolyploidy, Microsporogenesis, Pollen viability, Forage grass INTRODUCTION The improvement of Brachiaria grasses through breeding is complex and laborious. In this genus, the majority of species are polyploid (Basappa et al., 1987; Honfi et al., 1990; Bernini and Marin-Morales, 2001; Mendes-Bonato et al., 2002, 2006a; Utsunomiya et al., 2005) and polyploidy is correlated with apomixis (Valle and Savidan, 1996). Apomictics in this genus reproduce by apospory of the Panicum type and are pseudogamous, which means that the unreduced polar nucleus of the embryo sac needs to be fertilized for correct development of the endosperm (Alves et al., 2001). The two Brachiaria cultivars most widely used in Brazilian pastures, B. brizantha cv. Marandu and B. decumbens cv. Basilisk, are tetraploid and apomictic, and were directly selected through natural genetic variability. The major objective of the Brachiaria breeding program underway at the Embrapa Beef Cattle Research Center is the creation of new cultivars involving intra- and interspecific hybridization to introgress agronomic characteristics lacking in these cultivars, and also increase the genetic variability in the genus. To participate in a cross, the parental accessions both need to reproduce sexually, or the plant used as the female may reproduce sexually and the pollen donor by apomixis but with high pollen fertility. The first condition is difficult to accomplish in this genus because compatible sexual accessions with desirable agronomic characters are rare or non-existent. The second, on the other hand, has been extensively employed in several crosses where artificially tetraploidized sexual accessions of B. ruziziensis are crossed with apomictic genotypes of two closely related species, B. brizantha and B. decumbens. The success of hybridization, however, depends on the pollen fertility of the apomictic accession (Valle and Savidan, 1996; Miles et al., 2004). Cytological characterization of accessions of B. brizantha from the Embrapa Beef Cattle germplasm collection has revealed accessions with a low frequency of meiotic abnormalities (Mendes-Bonato et al., 2002, 2006a; Utsunomiya et al., 2005). Nonetheless, several accessions have shown abnormalities besides those typical of polyploidy (Mendes-Bonato et al., 2001a,b; Risso-Pascotto et al., 2003; Mendes, 2004). The present study reports abnormalities observed in one accession of B. brizantha (B176), including an abnormality never before reported in any other Brachiaria species. MATERIAL AND METHODS The accession B176 (BRA004189) of B. brizantha from the Embrapa Beef Cattle germplasm collection (Campo Grande, State of Mato Grosso do Sul, Brazil) which was collected in the wild-African savannas in the 1980s by CIAT (Colombia) was thoroughly analyzed by light microscopy. Site characteristics of cultivation in the field in Brazil were: climate type Aw tropical humid savanna; average annual precipitation = 1526 mm; average temperature = 22°C; altitude = 520 m; latitude = 20° 28’ S; longitude = 55° 40’ W; soil poor dark red latossol (59% sand; 8% silt; 33% clay; pH = 4.2). Inflorescences for meiotic study were collected from a single plant representing the accession and fixed in a mixture of 95% ethanol, chloroform and propionic acid (6:3:2) for 24 h. They were then transferred to 70% alcohol and stored, refrigerated until use. Microsporocytes were prepared by squashing and staining with 0.5% propionic carmine. Photomicrographs were taken with a Wild Leitz microscope using Kodak Imagelink - HQ, ISO 25 black and white film. RESULTS AND DISCUSSION Due to its interesting agronomic characteristics, the B. brizantha germplasm collection at Embrapa Beef Cattle is represented by 233 accessions collected from different countries in eastern Africa. According to flow cytometry indices determined for 222 of these accessions (Penteado et al., 2000), the great majority of them (220 accessions) are polyploid. The accession B176, whose cytometry index indicated a ploidy level of 5n (2n = 5x = 45) is, actually, a hexaploid accession (2n = 6x = 54) with x = 9, the predominant basic chromosome number in the genus Brachiaria followed by x = 7 (Basappa et al., 1987; Honfi et al., 1990; Bernini and Marin-Morales, 2001; Mendes-Bonato et al., 2002, 2006a; Utsunomiya et al., 2005). The percentage of cells with meiotic abnormalities related to polyploidy, including precocious chromosome migration and laggards generating micronuclei (Figure 1) was high as expected in this accession (Table 1) considering its hexaploid condition. In several other polyploid accessions of B. brizantha previously analyzed different frequencies of meiotic abnormalities related to polyploidy have been recorded (Mendes-Bonato et al., 2002; Risso-Pascotto et al., 2003; Mendes, 2004). Chromosome stickiness, also observed in some cells in this accession, has been described in B. brizantha (Mendes-Bonato et al., 2001a; Mendes, 2004) and in other Brachiaria species (Mendes-Bonato et al., 2001c; Utsunomiya et al., 2005).
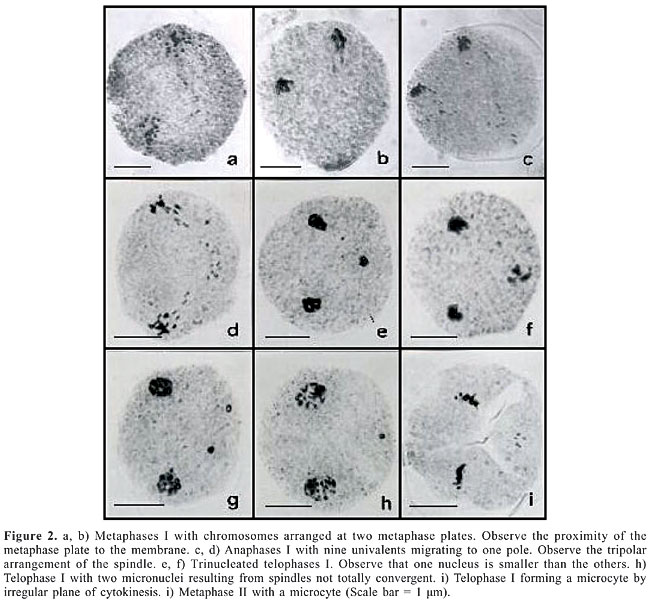
In this accession, one cytological characteristic differentiated it from the other hexaploid accessions of B. brizantha previously analyzed (Mendes-Bonato et al., 2002; Mendes, 2004). In the latter, chromosomes were always arranged at a single-metaphase plate and showed normal segregation at anaphase I. In B176, however, nearly 40% of meiocytes (Table 1) had the chromosome set arranged at two metaphase plates (Figure 2), while the remainder of the cells were normal (Figure 1). In the abnormal cells, the two chromosome sets were arranged at metaphase plates that occupied a polar site place close to the membrane (Figure 2a to d). The two metaphase plates did not show a parallel arrangement, but instead were arranged at an angle to form a typical tripolar spindle (Figure 2a to d). These cells did not show normal chromosome segregation at anaphase I. Only a few chromosomes migrated from each metaphase plate to the opposite pole with the remainder staying stationary in the plate. Chromosome counts in the small segregated group, suggested that nine univalent chromosomes migrated to opposite poles (Figure 2d). As a result of such spindle orientation and chromosome behavior, telophases I with three nuclei, with one of them being smaller, were recorded (Figure 2e, f). In some cells, the spindles were not totally convergent and two small nuclei were observed in telophases I (Figure 2g). Cytokinesis eliminated the small nuclei into a microcyte after telophase (Figure 2h, i). The second division proceeded normally, with the presence of microcytes in all phases.
The origin of such an abnormality could be related to the hexaploid nature of this accession. Hexaploidy might have originated by chromosome doubling of a triploid which, on the other, could have originated from the union of an n gamete (n = 9, sexual parent) and an unreduced gamete (2n = 18, apomictic parent). The genitors of the triploid could be of different species or of wide genetic origin that did not display the same behavior for spindle organization. Chromosome doubling in triploids occurs in order to restore the fertility through the correct chromosome pairing. However, in this accession, chromosomes were separated after doubling, and therefore, fertility could not be re-established. It is interesting that only the nine univalent chromosomes of each triploid set migrated to the opposite pole and the other genome remained unsegregated. The combination of two genomes in an interspecific hybrid frequently results in aberrant meiotic divisions, including those involving spindle orientation. Evidence of natural hybridization in a B. brizantha accession with genomes not closely related was first observed by Mendes et al. (2006). Also abnormal spindle orientation has been reported in an artificially tetraploidized accession of B. ruziziensis (Risso-Pascotto et al., 2005). The absence of genome affinity in relation to chromosome distribution in the metaphase plate has been recorded in an interspecific hybrid between an sexual artificially tetraploidized sexual accession of B. ruziziensis and B. brizantha (Mendes-Bonato et al., 2006b). In that case, both parental genomes were separated on two metaphase plates that behaved asynchronously during microsporogenesis. Relatively little is known about the development of the meiotic spindle in plants. It is known, however, that after the nuclear envelope breaks down, randomly oriented microtubule bundles in the cytoplasm are seen assuming a bipolar orientation and form an anastral spindle (Hepler et al., 1993). In higher plant cells devoid of centrioles, the centrosome has not yet been identified as a morphological structure and the mechanisms of anastral division spindle formation remain unclear (Shamina et al., 2000). There is no information in the literature about the influence of genetic factors on spindle formation. However, taking into account that the equilibrium for bipolar spindle formation is generally disrupted in hybrids, it is plausible that this character is genetically controlled, and that the union of divergent genomes in a common cytoplasm could disrupt the abnormal spindle orientation, as observed in the present hexaploid accession of B. brizantha. Because of its high percentage of meiotic abnormalities, this accession is ineligible to participate in crosses and may be discarded from the breeding program. REFERENCES Alves ER, Carneiro VT and Araújo AC (2001). Direct evidence of pseudogamy in apomictic Brachiaria brizantha (Poaceae). Sex. Plant Reprod. 4: 207-212. Basappa GP, Muniyamma M and Chinnappa CC (1987). An investigation of chromosome numbers in the genus Brachiaria (Poaceae: Paniceae) in relation to morphology and taxonomy. Can. J. Bot. 65: 2297-2309. Bernini C and Marin-Morales MA (2001). Karyotype analysis in Brachiaria (Poaceae) species. Cytobios 104: 157-171. Hepler PK, Cleary AL, Gunning BE, Wadswordth P, et al. (1993). Cytoskeletal dynamics in living plant cells. Cell. Biol. Int. 17: 127-142. Honfi AI, Quarin CL and Valls JF (1990). Estudios cariologicos en gramineas sudamericanas. Darwiniana 30: 87-94. Mendes DV (2004). Avaliação citogenética de acessos de Brachiaria brizantha (Gramineae). Master’s thesis in Genetics and Improvement, Universidade Estadual de Maringá, Maringá. Mendes DV, Boldrini KR, Mendes-Bonato AB, Pagliarini MS, et al. (2006). Cytological evidence of natural hybridization in Brachiaria brizantha Stapf (Gramineae). Bot. J. Linn. Soc. 150: 441-446. Mendes-Bonato AB, Pagliarini MS, Silva N and Valle CB (2001a). Meiotic instability in invader plants of signal grass Brachiaria decumbens Stapf (Gramineae). Acta Scientiarum 23: 619-625. Mendes-Bonato AB, Pagliarini MS, Valle CB and Penteado MI (2001b). A severe case of chromosome stickness in pollen mother cells of Brachiaria brizantha (Hochst) Stapf (Gramineae). Cytologia 66: 287-291. Mendes-Bonato AB, Pagliarini MS, Valle CB and Penteado MI (2001c). Archesporial syncytes restricted to male flowers in a hexaploid accession of Brachiaria brizantha (Hochst) Stapf (Gramineae). Nucleus 44: 137-140. Mendes-Bonato AB, Pagliarini MS, Forli F, Valle CB, et al. (2002). Chromosome number and microsporogenesis in Brachiaria brizantha (Gramineae). Euphytica 125: 419-425. Mendes-Bonato AB, Pagliarini MS, Risso-Pascotto C, Valle CB, et al. (2006a). Chromosome number and meiotic behavior in Brachiaria jubata (Gramineae). J. Genet. 85: 83-87. Mendes-Bonato AB, Risso-Pascotto C, Pagliarini MS and Valle CB (2006b). Cytogenetic evidence for genome elimination during microsporogenesis in interspecific hybrid between Brachiaria ruziziensis and Brachiaria brizantha (Poaceae). Genet. Mol. Biol. 29: 711-714. Miles JW, Valle CB, Rao I and Euclides VP (2004). Brachiaria grasses. In: Warm-season (C4) grasses (Sollenberg L and Burson B, eds.). Agronomy Monograph, 45. ASA-CSSA-SSSA, Madison, 745-783. Penteado MI, Santos AC, Rodrigues IF, Valle CB, et al. (2000). Determinação de poliploidia e avaliação da quantidade de DNA total em diferentes espécies de gênero Brachiaria. Boletim de Pesquisa, 11. Embrapa Gado de Corte, Campo Grande, 19. Risso-Pascotto C, Pagliarini MS, Valle CB and Mendes-Bonato AB (2003). Chromosome number and microsporogenesis in pentaploid accession of Brachiaria brizantha (Gramineae). Plant Breed. 122: 136-140. Risso-Pascotto C, Pagliarini MS and do Valle CB (2005). Multiple spindles and cellularization during microsporogenesis in an artificially induced tetraploid accession of Brachiaria ruziziensis (Gramineae). Plant Cell Rep. 23: 522-527. Shamina N, Dorogova N and Trunova S (2000). Radial spindle and the phenotype of the maize meiotic mutant, dv. Cell Biol. Int. 24: 729-736. Utsunomiya KS, Pagliarini MS and do Valle CB (2005). Microsporogenesis in tetraploid accessions of Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Biocell 29: 295-301. Valle CB and Savidan YH (1996). Genetics, cytogenetics, and reproductive biology of Brachiaria. In: Brachiaria: biology, agronomy, and improvement (Miles JW, Maass BL and Valle CB, eds.). Centro Internacional de Agricultura Tropical, Cali, Colômbia/Empresa Brasileira de Pesquisa Agropecuária, Brasília, 147-163. |
|