ABSTRACT. Microsporogenesis was analyzed in five accessions of Brachiaria dictyoneura presenting x = 6 as the basic chromosome number. All accessions were tetraploid (2n = 4x = 24) with chromosome pairing in bi-, tri-, and quadrivalents. The recorded meiotic abnormalities were those typical of polyploids, including precocious chromosome migration to the poles, laggard chromosomes, and micronucleus formation. The frequency of these abnormalities, however, was lower than those reported for other polyploid accessions previously analyzed for other Brachiaria species. Cell fusion and absence of cytokinesis were also recorded in some accessions, leading to restitutional nucleus formation in some cells. Genetically unbalanced microspores, binucleate, and 2n microspores were found among normal meiotic products as results from these abnormalities. The limitation in using these accessions as pollen donor in interspecific crosses with sexual species with x = 7 or x = 9 in breeding programs is discussed. Key words: Brachiaria dictyoneura, Interspecific hybridization, Basic chromosome number, Microsporogenesis, Cell fusion, Cytokinesis INTRODUCTION The genus Brachiaria includes both annual and perennial species. Amongst the perennials, B. dictyoneura is important as forage in the humid and subhumid tropics (Lascano and Euclides, 1996). It has been used for sown pastures and it was compared to B. humidicola from which it differs by its growth habit: tufted and not stoloniferous as the last (Renvoize et al., 1996). In Colombia, B. dictyoneura cv Llanero was released by the Instituto Colombiano Agropecuario in 1987. This cultivar was derived from seeds introduced from Australia (CSIRO) but was originally collected in Zambia in 1971. It is well adapted to low-fertility acid soils and tolerates some poor drainage. Its nutritive value is comparable to commercial B. humidicola. It is tolerant, but not resistant, to spittlebugs (Keller-Grein et al., 1996). In Brazil, this cultivar had only a relative success due to its slight advantages over the generally sown B. humidicola. There are no commercial cultivars of B. dictyoneura on the market until today. Some Brachiaria species with agronomic potential are inadequately represented in the existing collections around the world. No more than 15 accessions of B. dictyoneura are found in the major germplasm collections. In the Brazilian Brachiaria collection at Embrapa Beef Cattle (Campo Grande, MS), only nine accessions are available. Some of these accessions have shown great potential as source of genetic variability to be explored in interspecific crosses. Considering that the majority of the accessions of Brachiaria analyzed so far is polyploid and apomictic, and that the breeding program is restricted to sexual plants showing the same ploidy level as the pollen donors, the cytogenetic characterization of accessions of this collection is essential to direct the choice of the progenitors in the hybridization programs. Preliminary studies performed in B. dictyoneura by Risso-Pascotto et al. (2006) showed a new basic chromosome number (x = 6) for the genus Brachiaria. The current paper reports a detailed study on the microsporogenesis of five promising accessions from the Embrapa Brachiaria collection. MATERIAL AND METHODS Five accessions of B. dictyoneura (BRA005851, BRA007871, BRA007889, BRA007897, and BRA007919) from the Embrapa Beef Cattle Brachiaria collection (Campo Grande, Mato Grosso do Sul State, Brazil) were cytologically analyzed. Inflorescences for meiotic study were collected from plots of 16 plants representing each accession and fixed in a mixture of 95% ethanol, chloroform and propionic acid (6:3:2) for 24 h, transferred to 70% alcohol and stored under refrigeration until use. Slides of microsporocytes were prepared by squashing and staining with 0.5% propionic carmine. Chromosome association at diakinesis was evaluated in 20 meiocytes per accession, and more than 110 meiocytes per phase, from metaphase I to tetrad, were investigated in their meiotic behavior. Photomicrographs were made with a Wild Leitz microscope using Kodak Imagelink - HQ, ISO 25 black and white film. RESULTS AND DISCUSSION Chromosome countings during microsporogenesis (Figure 1a, b, and d) showed that all accessions presented 2n = 24 chromosomes. This genus has been characterized as having two basic chromosome numbers, x = 7 and x = 9, with the predominance of the latter (Basappa et al., 1987; Honfi et al., 1990; Morrone and Zuloaga, 1992; Valle and Savidan, 1996; Utsunomiya et al., 2005; Mendes-Bonato et al., 2002a, 2006). Brachiaria dictyoneura and B. humidicola are closely related and, at times, have been erroneously identified by agronomists. Two basic chromosome numbers, x = 7 and x = 9, have been reported for B. humidicola (Bernini and Marin-Morales, 2001), suggesting that x = 6 found in B. dictyoneura by Risso-Pascotto et al. (2006) might have arisen from x = 7. However, the origin of this new basic chromosome number needs to be clarified in a detailed karyological study.
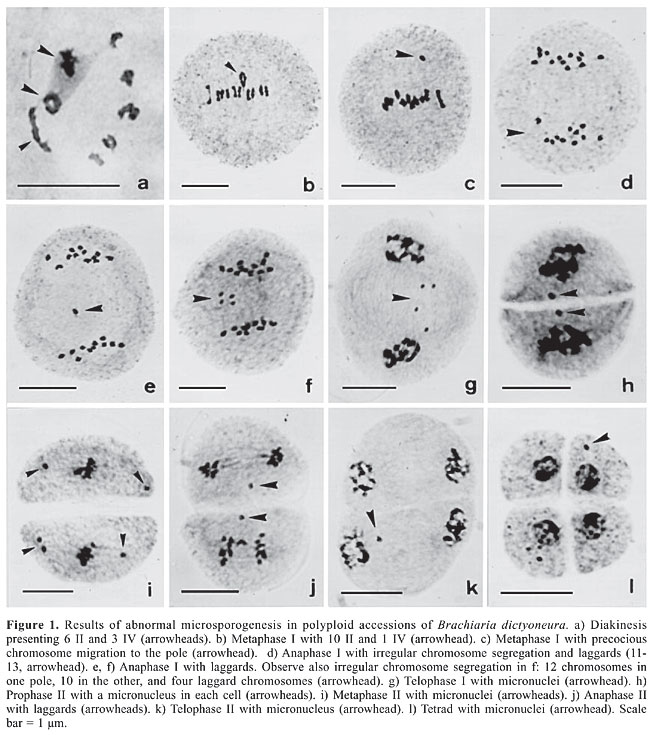
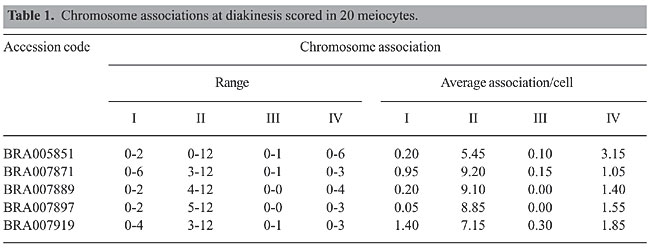
Chromosome pairing at diakinesis was typical of tetraploidy (2n = 4x = 24). Chromosome was associated predominantly as bivalents, but uni-, tri, and quadrivalents were also recorded (Table 1; Figure 1a and b). Such chromosome pairing is expected based on random associations of four homologous chromosomes, i.e., in autotetraploids (Singh, 1993). In the accession BRA005851, the frequency of quadrivalents was higher than that in the other accessions (Table 1). In some cells, the 24 chromosomes were associated as six quadrivalents, suggesting that the four genomes are homologous.
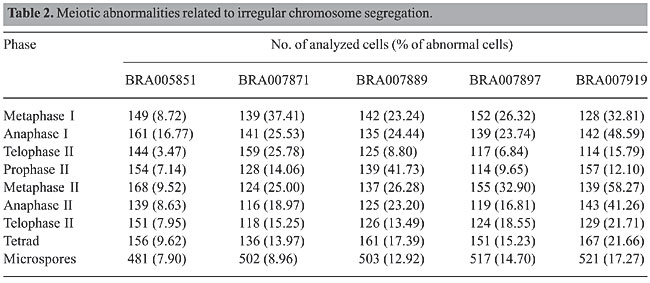
The overall meiotic behavior in these accessions showed to be typical of polyploids (Table 2) with irregular chromosome segregation in both divisions. The accession BRA005851, which presented chromosomes associated mainly as bivalents or quadrivalents, showed lower frequencies of abnormal meiocytes. Figure 1 illustrates the meiotic abnormalities recorded in the accessions. Precocious chromosome migration to the poles in metaphases (Figure 1c and i), laggards in anaphases (Figure 1e, f, and j), micronuclei in telophases (Figure 1g and k), prophase II (Figure 1h), and tetrads (Figure 1 l) were the main abnormalities. The percentage of abnormal tetrads among accessions ranged from 9.62% in BRA005851 to 21.66% in BRA007919. Abnormalities such as these, but in much higher frequencies, have been previously reported for polyploid accessions of different species of Brachiaria (Risso-Pascotto et al., 2003; Utsunomiya et al., 2005; Mendes-Bonato et al., 2002a, 2006) and are responsible for pollen sterility due to unbalanced microspores.
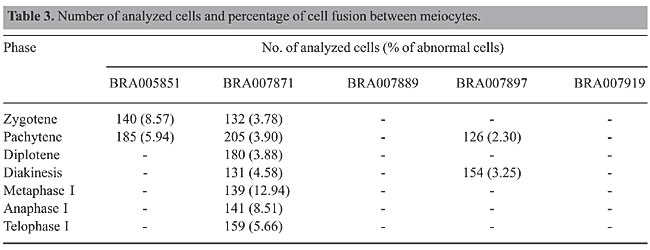
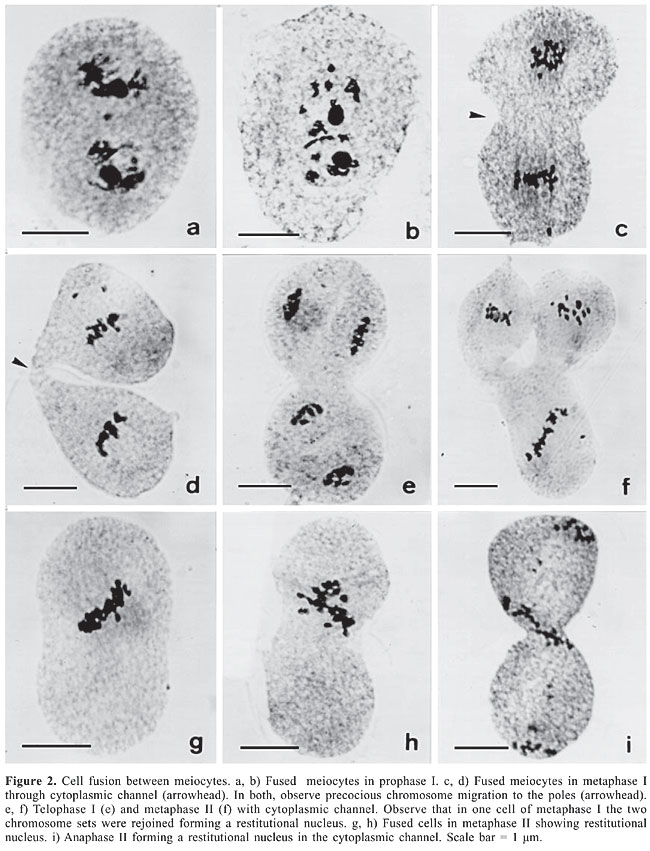
Cell fusion detected in three B. dictyoneura accessions (Table 3) was also reported for other Brachiaria species (Mendes-Bonato et al., 2001, 2002b, 2003; Risso-Pascotto et al., 2003; Utsunomiya et al., 2004, 2005). In the present accessions, only two or three cells were involved in each fusion, whereas in the other Brachiaria species up to ten cells were recorded forming syncytes. As reported for other species of Brachiaria, in the present accessions, cell fusions reported in the other Brachiaria species are more common in prophase I, when the callose wall around the meiocyte is not completely formed. In B. dictyoneura, we could distinguish two types of cell fusion: completely fused cells, in which the genomes were in the same cytoplasm (Figure 2a and b), and those connected by a thin or a thick cytoplasmic channel (Figure 2c to i). In some meiocytes, the proximity of genomes due to cell fusion led to the formation of a restitutional nucleus by the rejoining of the chromosomes (Figure 2f to i). If the meiotic process evolves normally in the fused cells, restitutional nuclei will give rise to 2n gametes. Despite the majority of cells being connected by cytoplasmic channels typical of those recorded during cytomixis, chromosome transfer between meiocytes was not detected in B. dictyoneura. Similar cytoplasmic channels promoted cytomixis in tetraploid accessions of B. nigropedata (Utsunomiya et al., 2004).
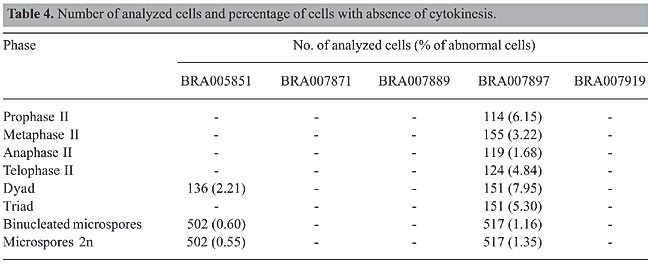
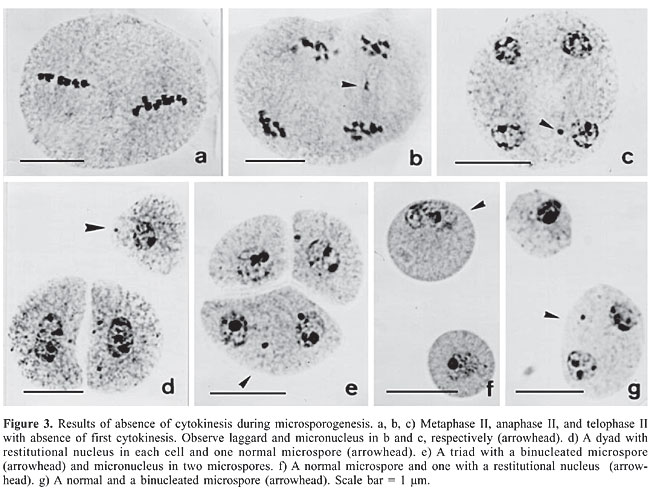
Absence of first or second cytokinesis was also recorded in two accessions of B. dictyoneura, but the frequency was much higher in BRA007897 (Table 4, Figure 3). When failure of cytokinesis occurred only in the first division (Figure 3a to c), with normal cytokinesis in the second division, a dyad of microspores was formed (Figure 3d). The two nuclei of each cell in the dyad followed one of two distinct ways: they remained isolated, giving rise to binucleated microspores (Figure 3g) or they were rejoined, originating a restitutional nucleus that developed into a 2n gamete (Figure 3d and f). When the first division was normal and the failure of cytokinesis occurred only in the second division, dyads and triads were formed if the failure affected one or both cells. Triads (Figure 3e) were formed when cytokinesis occurred in only one cell of the dyad. The frequency of binucleated microspores and of 2n microspores was similar in each accession, BRA005851 or BRA007897, but were higher in accession BRA007897.
Absence of cytokinesis leading to 2n gamete formation has been reported in many plant species (Veilleux, 1985), and also in B. brizantha (Risso-Pascotto et al., 2003). 2n gametes played an important role in evolution through polyploidization and in plant breeding (Veilleux, 1985; Bretagnolle and Thompson, 1995). The majority of Brachiaria species is polyploid and reproduces by apomixis which, by circumventing meiosis and fertilization in the megagametophyte followed by parthenogenetical production of a non-reduced embryo, indefinitely preserves the genetic constitution of the mother plant (Valle and Savidan, 1996). The meiotic behavior in polyploid accessions of Brachiaria species studied by Mendes-Bonato et al. (2002a, 2006) and Utsunomiya et al. (2005) provided evidence that polyploidy in this genus may have been originated predominantly by chromosome doubling instead of interspecific hybridization. In this context, 2n gamete could have played a fundamental role in the evolutionary history of the genus. The main objective of the Brachiaria breeding program is to produce fertile hybrids by interspecific hybridization, combining alleles for important agronomic traits, thus increasing the genetic variability of these plants and new options for forage cultivars. Crosses between Brachiaria species demand compatibility between progenitors: sexual accessions as female genitor and apomictic pollen donor with the same basic chromosome number and ploidy level. Sexuality, in the Brachiaria agamic complex that is being bred, has been reported mainly for diploid accessions with basic chromosome number x = 9, particularly in B. ruziziensis (Valle and Savidan, 1996). However, previous studies of the reproduction mode of the present B. dictyoneura accessions showed one of them (BRA007897) as sexual (Valle CB, unpublished data). The present results of cytogenetic analyses and the new basic chromosome number x = 6 limit the use of these accessions as pollen donors in crosses with other sexual Brachiaria species with x = 7 or x = 9, particularly to the taxonomically related B. humidicola x = 9. However, the sexual tetraploid accession of B. dictyoneura (2n = 4x = 24) can be used as maternal genitor in crosses with the other apomictic tetraploid accessions under analyses with the same basic chromosome number. Continued cytogenetic studies of the Brachiaria collection are essential for the inclusion of these accessions as pollen source in the interspecific hybridization program. For that, new sources of compatible sexual species with the same basic chromosome number need to be identified. REFERENCES Basappa GP, Muniyamma M and Chinnappa CC (1987). An investigation of chromosome numbers in the genus Brachiaria (Poaceae: Paniceae) in relation to morphology and taxonomy. Can. J. Bot. 65: 2297-2309. Bernini C and Marin-Morales MA (2001). Karyotype analysis in Brachiaria (Poaceae) species. Cytobios 104: 157-171. Bretagnolle F and Thompson JD (1995). Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 129: 1-22. Honfi AI, Quarin CL and Valls JFM (1990). Estudios cariologicos en gramineas sudamericanas. Darwiniana 30: 87-94. Keller-Grein G, Maass BL and Hanson J (1996). Natural variation in Brachiaria and existing germplasm collection. In: Brachiaria: biology, agronomy and improvement (Miles JW, Maass BL and Valle CB, eds.). CIAT/Embrapa, Colombia, 17-42. Lascano CE and Euclides VPB (1996). Nutritional quality and animal production of Brachiaria pastures. In: Brachiaria: biology, agronomy and improvement (Miles JW, Maass B and Valle CB, eds.). CIAT/Embrapa, Colombia, 106-123. Mendes-Bonato AB, Pagliarini MS, Valle CB and Penteado MIO (2001). Meiotic instability in invader plants of signal grass Brachiaria decumbens Stapf (Gramineae). Acta Sci. 23: 619-625. Mendes-Bonato AB, Pagliarini MS, Forli F, Valle CB, et al. (2002a). Chromosome number and microsporogenesis in Brachiaria brizantha (Gramineae). Euphytica 125: 419-425. Mendes-Bonato AB, Pagliarini MS, Valle CB and Penteado MIO (2002b). Archesporial syncytes restricted to male flowers in a hexaploid accession of Brachiaria brizantha (Hochst) Staph (Gramineae). Nucleus 45: 1-5. Mendes-Bonato AB, Pagliarini MS, Risso-Pascotto C and Valle CB (2003). Normal microspore production after cell fusion in Brachiaria jubata (Gramineae). Genet. Mol. Biol. 26: 517-520. Mendes-Bonato AB, Pagliarini MS, Risso-Pascotto C and Valle CB (2006). Chromosome number and meiotic behavior in Brachiaria jubata (Gramineae). J. Genet. 85: 83-87. Morrone O and Zuloaga FO (1992). Revision de las espécies sudamericanas nativas e introducidas de los generos Brachiaria e Urochloa (Poaceae: Panicoideae: Paniceae). Darwiniana 31: 43-109. Renvoize SA, Clayton WD and Kabuye CHS (1996). Morphology, taxonomy, and natural distribution of Brachiaria (Trin.) Griseb. In: Brachiaria: biology, agronomy and improvement (Miles JW, Maass BL and Valle CB, eds.). CIAT/Embrapa, Colombia, 1-15. Risso-Pascotto C, Pagliarini MS, Valle CB and Mendes-Bonato AB (2003). Chromosome number and microsporogenesis in pentaploid accession of Brachiaria brizantha (Gramineae). Plant Breed. 122: 136-140. Risso-Pascotto C, Pagliarini MS and Valle CB (2006). A new basic chromosome number for the genus Brachiaria (Trin.) Griseb. (Poaceae: Panicoideae: Paniceae). Genet. Res. Crop Evol. 53: 7-10. Singh RJ (2006). Plant cytogenetics. CRC Press Inc., Boca Raton. Utsunomiya KS, Pagliarini MS and do Valle CB (2004). Chromosome transfer among meiocytes in Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Cytologia 69: 395-398. Utsunomiya KS, Pagliarini MS and do Valle CB (2005). Microsporogenesis in tetraploid accessions of Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Biocell 29: 295-301. Valle CB and Savidan YH (1996). Genetics, cytogenetics, and reproductive biology of Brachiaria. In: Brachiaria: biology, agronomy and improvement (Miles JW, Maass BL and Valle CB, eds.). CIAT/Embrapa, Colombia, 147-163. Veilleux R (1985). Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breed. Rev. 3: 253-288. |
|