
|
Prevalence of vitamin D receptor gene polymorphism in a Uruguayan population and its relation to type 1 diabetes mellitus A. Mimbacas1,2, J. Trujillo2*, C. Gascue2*, G. Javiel3,4* and H. Cardoso2* 1Departamento de Citogenética, Unidad Asociada Instituto de Biología,Facultad de Ciencias, Montevideo, Uruguay 2Departamento de Genética, Instituto de Investigaciones Biológicas Clemente Estable, Montevideo, Uruguay 3Unidad de Diabetes, C.A.S.M.U., Montevideo, Uruguay 4Hospital Pasteur, MSP, Montevideo, Uruguay *These authors contributed equally to this study. Corresponding author: A. Mimbacas E-mail: [email protected] Genet. Mol. Res. 6 (3): 534-542 (2007) ABSTRACT. Vitamin D has important immuno-modulatory properties and it influences insulin secretion. It acts through a vitamin D receptor (VDR), for which several gene polymorphisms have been described. The Uruguayan population presents several epidemiological characteristics that make it different from that of other counties, including other Latin-American countries. It went through miscegenation processes, with a tri-hybrid European, Amerindian and African origin, with no contribution from isolated Amerindian communities. Such differences have important consequences for the relationship between frequencies of several genes in the general population and their association with the diabetes mellitus. We examined the prevalence of VDR gene polymorphisms in the general population and their relation to type 1 diabetes in a parent-case design. One hundred unrelated individuals from the general population and 45 parent-patient triads with a child affected with type 1 diabetes were genotyped for FokI, BsmI and TaqI VDR gene polymorphisms by RFLP-PCR. We used a transmission disequilibrium test to assess preferential transmission of parents to affected offspring. The prevalence of the three VDR polymorphisms was: allele F = 48%, B = 35%, T = 64%. The f, b, T alleles and heterozygous genotypes were found at a high frequency in this population. Among 36 informative heterozygous parental genotypes, 30 transmitted the F allele (probability of transmission = 83%). The other two polymorphisms did not show significant transmission. We suggest that FokI polymorphism indicates susceptibility to type 1 diabetes mellitus in the Uruguayan population. Key words: Vitamin D receptor polymorphisms, Ethnic origin, Prevalence, Transmition/disequilibrium test INTRODUCTION The Uruguayan population is a mixture of three ethnic groups: European, Amerindian and African. Unlike other Latin-American countries, there are no isolated Amerindian communities (Sans et al., 1997) and Uruguay went through an intense miscegenation process between all ethnic groups. This fact is important to analyze the behavior of this gene because in various previous studies we found differences in prevalence in the ancestor populations (Poggio et al., 2000; Mimbacas et al., 1998, 2003, 2004; Cardoso et al., 2004; Zorrilla et al., 2006, and Echarte L (personal communication). Moreover, our population has only three million people and half of them live in capital city; consequently, it is impossible to separate the population according to ethnic background. Global incidence of diabetes mellitus has been increasing in recent decades. There are strong differences between different geographical areas and population groups (IDFDA, 2003). This disease currently affects 8 in every 100 people in Uruguay (Ferrero and Garcia, 2005). This sudden increment suggests that besides genetic factors, the environment is also important. The main genes involved in susceptibility to type 1 diabetes mellitus (T1DM) are located in the major histocompatibility complex in human leukocyte antigen (HLA) genes (Verge and Eisenbarth, 1996; Gorodezky et al., 1997; Ronningen, 1997; Ettinger et al., 1998). In Uruguay, a clear association has been found between some HLA groups and T1DM (Mimbacas et al., 1998, 2003, 2004). Additional genetic markers along with environmental factors have been suggested as indicative of susceptibility to T1DM (Cordell and Todd, 1995; Akerblom et al., 2002). The vitamin D receptor gene (VDR) has been considered as a possible risk factor for T1DM. Mathieu et al. (1992) demonstrated that treatment with large doses of vitamin D over a long period in NOD mice prevents the disease. After that, the Eurodiab Substudy 2 Study Group (1999) and Hyppönen et al. (2001) found that the vitamin D supplementation in infancy is associated with a lower risk of developing T1DM. Norman et al. (1980) demonstrated that vitamin D deficiency inhibits the secretion of pancreatic insulin. Vitamin D acts through union with its specific receptor, a nuclear protein called VDR (Reichel et al., 1989; Cantorna, 2000). Several polymorphisms have been described for the VDR gene (Farrow, 1994; Verbeek et al., 1997; Gross et al., 1998; Jurutka et al., 2000). The frequencies of these polymorphisms vary in different populations; until now association studies have produced contradictory results (McDermott et al., 1997; Hauache et al., 1998; Chang et al., 2000; Pani et al., 2000; Ban et al., 2001; Guja et al., 2002; Györffy et al., 2002; Yokota et al., 2002; Turpeinen et al., 2003; Motohashi et al., 2003; Angel et al., 2004; Marti et al., 2004; Nejentsev et al., 2004; Capoluongo et al., 2006). We examined the prevalence of FokI, BsmI and TaqI VDR gene polymorphisms in a sample of individuals that is representative of the general population in Uruguay. As our country has a highly mixed population we considered that the most appropriate approach to look for a possible association between VDR polymorphisms and TIDM is a case-parent triad design. MATERIALS ANS METHODS Prevalence study Based on epidemiological and statistical studies we estimated the representative n value for genetic studies of our population. We extracted DNA samples from 500 individuals to construct the DNA banks. The subjects were selected at random among unrelated individuals attended at public and private medical health institutions. Data from a previous study (Cardoso et al., 2004) allowed us to stratify the population at each health center into six arbitrary socio-economic categories and determine their corresponding frequencies. The number of subjects enlisted at each institution was established based on these socio-economic categories. This study (Ref. 1081/96) was approved by the Public Health Ministry (Uruguay). Knowing the frequency of diabetes mellitus (8/100) we examined a representative sample of DNA (101 individuals). Case-parent design To study the possible association between VDR polymorphisms and T1DM, 45 children affected with T1DM and their parents were selected for this study. The transmission/disequilibrium test (TDT) was used to detect preferential transmission from heterozygous parents to an affected offspring (Spielman et al., 1993). The following criteria were considered to include patients in this study: a) age under 15 years, b) the possibility of studying both parents, c) the diabetic patients were considered as affected by T1DM according to American Diabetes Association criteria (Report ADA, 2003). The cases attended public and private health centers. All the participants were informed of this study, and agreed to participate by written consent. Genomic DNA was extracted from peripheral blood by phenol-chloroform technique or with DNAzol (Promega). The genotypes were analyzed with polymerase chain reaction followed by restriction fragment length polymorphism analysis (PCR/RFLPs) to define three polymorphic sites: BsmI, TaqI and FokI. We designated with lower case letters the presence of the restriction site for the enzyme and capital letters for its absence. All samples were analyzed and validity of genotyping ensured. The frequencies of alleles were calculated from the genotype data by the gene count method. The chi-square test was used to check for Hardy-Weinberg equilibrium and to evaluate differences between the distribution of VDR polymorphisms of the general population and that of other ethnic groups. Given the small sample size and sparse data in the transmission tables, global and allele-specific P values for association using TDT were computed through exact methods implemented in the statistical package STATA 7.0 (Stata Corporation, College Station, TX, USA, 2001) and SPSS version 10.0 for Windows (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered to be significant. RESULTS The VDR polymorphisms for all individuals were determined using PCR-RFLP methodology. Prevalence data were estimated for the alleles and genotypes for FokI, BsmI and TaqI polymorphisms in the general population (Tables 1 and 2). The most frequently observed haplotypes were: FfBbTt (26.5%) and FfbbTT (24.10%). The distribution of genotypes for FokI and BsmI was significantly different from expectation under Hardy-Weinberg equilibrium, but not for TaqI polymorphism. In comparison with other populations, FokI polymorphism was significantly different (Table 2). However, BsmI and TaqI polymorphism frequencies were similar to French and African-American figures. In the T1DM families, the most frequent genotype in diabetic patients was FFbbTT (25%). An estimated frequency of 83% of heterozygous genotypes for three VDR polymorphisms was informative to perform the TDT analysis. The most frequent parental genotype was FFBbTt (19%). Despite the fact that the haplotype FbT was preferentially transmitted, no significance in TDT analysis was found. We estimated preferential transmission from parents to affected offspring for the three polymorphisms in an independent manner (Table 3). Only the F allele was found to have significantly increased transmission; the other two polymorphisms did not. 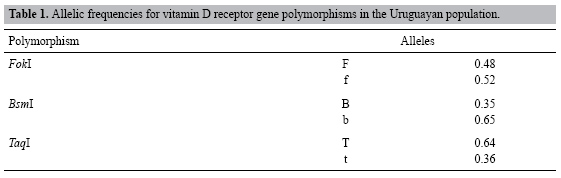 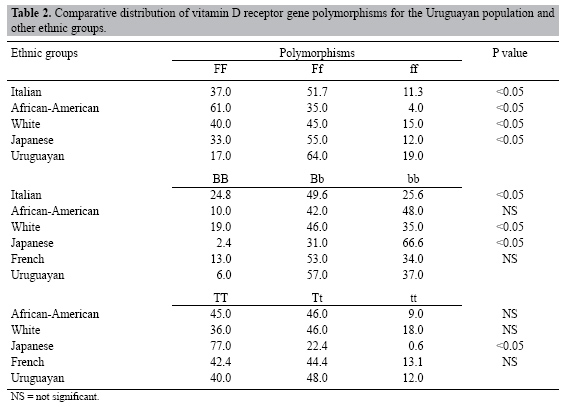  DISCUSSION It is important to determine the prevalence of different genes in our population before searching for association with a specific disease. Our population is a result of a mixture of European (86-96%), African (4-11%) and Amerindian (1-7%) ancestors (Sans et al., 1997). The European origin is mainly from Spain, Italy, France, and Portugal, but various other communities have lived here since the 18th century (including Jews, Armenians, and Lithuanians). Africans came from the coasts of Congo and Angola; they arrived during and following Portuguese-Hispanic colonization in the 16th century, while Amerindians belong to native groups (mainly "Charruas" and "Guaraníes") that existed previous to colonization. Admixture between all these different ethnic groups was intensive as there were no isolated groups. At present, Uruguay has 3.2 million inhabitants, and half of them live in the capital city, Montevideo. Furthermore, this makes our country different, even compared to other Latin-American countries. We have been examining candidate genes related to diabetes mellitus in our population. It is known that type 1 diabetes is a multi-factorial disease, with genetic and environmental factors that could explain the incidence rates that have been found in different ethnic groups and countries. Taking into consideration the environmental influence on development of this disease and its relation with genetic factors, we examined the VDR polymorphisms that interact with vitamin D. Vitamin D has important immune regulatory properties and its action influences insulin secretion (Norman et al., 1980; Cantorna et al., 1996, 1998; Cippitelli and Santoni, 1998; Jones et al., 1998; Cantorna, 2000; Kato, 2000; Overbergh et al., 2000; DeLuca and Cantorna, 2001; Hayes et al., 2003). We determined the distribution of the three VDR gene polymorphisms in the general population. Only the TaqI polymorphism was in Hardy-Weinberg equilibrium. One possible explanation for the deviations in the frequencies of the FokI and BsmI polymorphisms is that these polymorphic variants are directly or indirectly related to epigenetic mechanisms. The BsmI polymorphism could have some function in transcriptional regulation due to its 3’ untranslated region position (Farrow, 1994; Verbeek et al., 1997; Durrin et al., 1999). However, the FokI VDR polymorphism results in two isoforms of VDR protein. This polymorphism occurs at the first ATG start codon of VDR protein. The absence of the FokI site (denoted F) indicates that the first codon is ACG, resulting in translational initiation at an in-frame ATG, three codons downstream. Consequently, the FokI polymorphism produces either a 424- (F) or 427- (f) amino acid VDR protein. These two VDR isoforms interact differently with TFIIB at the N-terminal region; the F VDR isoform possesses more potent transcriptional activity (Jurutka et al., 2000). Another possibility is biased sampling or non-random mating in this population, but due to the way in which we selected our sample, this was not a factor. Furthermore, we considered that the equilibrium deviation was not a size or power sample problem because other polymorphisms studied in the same DNA sample did not show departures from Hardy-Weinberg equilibrium, including angiotensin-converting enzyme gene, HLA DQB1 and DRB1 alleles, cystic fibrosis transmembrane regulator gene, and 2CP cytochrome P450 polymorphisms (Poggio et al., 2000; Mimbacas et al., 1998, 2003, 2004; Cardoso et al., 2004; Zorrilla et al., 2006, and Echarte L (personal communication). Diabetes mellitus and associated markers vary among different ethnic groups. Knowing the ancestry of the Uruguayan population, we compared our results with those for relevant populations (Suarez et al., 1997; Habuchi et al., 2000; Ban et al., 2001; Taverna et al., 2002; Oakley-Girvan et al., 2004; Capoluongo et al., 2006) (Table 2). We found that the prevalence of the three VDR polymorphisms showed a particular distribution. However, the White and African-American population data are from the San Francisco-Oakland Bay area in the USA. The distribution of allele frequencies differed from the Uruguayan data, but the origins of these ethnic groups were also different. Therefore, it was not possible to make objective comparisons. The relationship between the vitamin D receptor gene and T1DM has been analyzed in several populations, but few articles present a case-parent design or are performed in admixed populations (McDermott et al., 1997; Chang et al., 2000; Pani et al., 2000; Ban et al., 2001; Guja et al., 2002; Györffy et al., 2002; Yokota et al., 2002; Turpeinen et al., 2003; Motohashi et al., 2003; Marti et al., 2004; Nejentsev et al., 2004; Capoluongo et al., 2006). In Latin American, only partial studies have been published on Brazilian and Chilean populations (Hauache et al., 1998; Angel et al., 2004). Marti et al. (2004) suggested that the F allele may increase T1DM susceptibility in Navarre and Barcelona populations. Another study that was developed with patients from the Lazio region showed a slight association between T1DM patients and the ff VDR genotype (Capoluongo et al., 2006). These two studies could be considered due to our European ethnic origin but these regions did not contribute much to our genetic pool (Sans et al., 1997). Angel et al. (2004) did not suggest any association of VDR alleles with the disease in an admixed American population (Amerindian and Caucasian) in Chile; but in their study FokI polymorphism was not analyzed. In our study, the case-parent design only showed preferential transmission for FokI polymorphism. The case-parent design gives a better approximation when studying genetic factors associated with a particular disease in admixed populations, since stratification of the population could act as a confounding variable, which could explain the contradictory results presented in the different studies. In summary, the genotypes of the VDR gene polymorphisms with highest prevalence are the heterozygote. It is important to determine the prevalence of the gene polymorphisms in the populations in addition to developing epidemiological studies in the search for genetic factors involved in multi-factorial disease. Finding genetic factors associated with disease in different ethnic origin populations and using appropriate methodologies for admixed populations are key to studying a multi-factorial disease such as T1DM. In the Uruguayan population, subjects with the FF genotype appear to be prone to developing this disease. ACKNOWLEDGMENTS The authors thank Dr. Francisco Pérez-Bravo of the Laboratory of Molecular Epidemiology, Institute of Nutrition and Food Technology, Chile University, Santiago, Chile, for his assistance. REFERENCES Akerblom HK, Vaarala O, Hyoty H, Ilonen J, et al. (2002). Environmental factors in the etiology of type 1 diabetes. Am. J. Med. Genet. 115: 18-29. |
|