
Differential characterization of holocentric chromosomes in triatomines (Heteroptera, Triatominae) using different staining techniques and fluorescent in situ hybridization A. Morielle-Souza and M.T.V. Azeredo-Oliveira Genet. Mol. Res. 6 (3): 713-720 (2007) ABSTRACT. A comparative study of holocentric chromosomes in the triatomine species Panstrongylus megistus, Rhodnius pallescens and Triatoma infestans was carried out in order to characterize heterochromatin, rDNA active sites and nucleolar proteins. Cytological preparations of seminiferous tubules were stained by silver impregnation, C banding, fluorochromes cma3/da and dapi/da, and fluorescent in situ hybridization (FISH) with Drosophila melanogaster 28S rDNA probe. Our results showed interesting aspects of the organization of chromatin and chromosomes in the meiotic cells of these insects. In R. pallescens, sex chromosomes (X, Y) were distinct from autosomes, when submitted to silver impregnation, C banding, CMA3 staining, and FISH, confirming that these chromosomes bear nucleolar organizer regions (NORs). In P. megistus, two of the three sex chromosomes were CMA3/DAPI-; at early meiotic prophase and at diakinesis, silver impregnation corresponded with FISH signals, indicating that in this species, two chromosomes (probably a sex chromosome and an autosome) bear NORs. In T. infestans, silver nitrate and FISH also stained corresponding areas on meiotic chromosomes. Our data suggest that in triatomines, in general, the number and location of NORs are species-specific. These regions may be considered important chromosome markers for comparative studies to improve the understanding of evolutionary mechanisms in these hematophagous insects. Key words: Holocentric chromosomes, Nucleolus organizer region, CMA3, DAPI, Fluorescent in situ hybridization, Triatomines INTRODUCTION Triatomines, hematophagous insect vectors of the protozoon Trypanosoma cruzi and causative agents of Chagas’ disease, are of great importance, not only in regard to the investigation of their biological control, but also because of their uncommon cytogenetic characteristics which include the presence of holocentric chromosomes and an unusual meiotic behavior with sex chromosomes segregating post-reductionally (Hughes-Schrader and Schrader, 1961; De Vaio et al., 1985). Until not very long ago, most cytogenetic studies on heteroptera holocentric chromosomes focused exclusively on heterochromatin and nucleolar organizer regions (NORs). However, molecular cytogenetic techniques, including fluorescent banding and fluorescent in situ hybridization (FISH) with specific probes, have allowed a better understanding of the constitution of chromatin in invertebrates, and thus have furthered the advance of studies in this group of animals. Although some heterochromatin is present on most eukaryotic chromosomes, its staining patterns and the nature of the DNA it contains vary widely within and among species. In addition, heterochromatin blocks are generally heteromorphic in relation to size and sometimes staining pattern. Differences among species with regard to heterochromatin location and amount are very frequent (Sumner, 1990). In some species, constitutive heterochromatin content varies greatly from individual to individual, and this variation may play an important role in speciation (Clark and Wall, 1996). In some organisms, such as fish and anurans, the number and location of rDNA/AgNOR are species-specific and important karyotypic markers. Indeed, silver impregnation, as well as FISH, have been demonstrated to be able to detect genomic NORs in these individuals (Fujiwara et al., 1998; Lourenço et al., 1998). Since information about the structure and base composition of some specific regions of triatomine holocentric chromosomes is relevant to the understanding of triatomine molecular structure, the aim of this study was to examine meiotic cells in species of the genera Panstrongylus, Rhodnius and Triatoma, by differential chromosome banding techniques (chromomycin A3/distamycin (CMA3/DA) and 4’6-diamycin-2-phenylindol-dihydrochloride/distamycin (DAPI/DA)), FISH with Drosophila melanogaster 28S rDNA probe, C banding and silver impregnation which reveal differential heterochromatin pattern and locate NORs as well as ribosomal DNA sites. MATERIAL AND METHODS Seminiferous tubules from adult insects of the following genera and species were used: Panstrongylus megistus (9A + X1X2Y), Rhodnius pallescens (10A + XY), and Triatoma infestans (10A + XY). Following the spread and fixation of the biological material (Imai et al., 1988), the slides were randomly submitted to the following analyses: silver impregnation (Howell and Black, 1980) for discrimination of nucleolar proteins; C banding (Sumner, 1972); double staining by fluorochromes CMA3/DA and DAPI/DA (Schmid, 1980) after pre-treatment with RNAse for heterochromatic discrimination, and FISH with D. melanogaster 28S-12-kb rDNA probe (Viegas-Péquignot, 1992) for rDNA localization. Photomicrographs were obtained using a Zeiss-Jenaval microscope for cytochemical techniques, and a Zeiss-Axioskop and Olympus BX-FLA for molecular cytogenetic techniques (CMA3/DA, DAPI/DA and FISH). RESULTS Panstrongylus megistusSilver-stained meiotic cells showed two chromosomes with NORs in spermatogonial metaphases. In nuclei at early meiotic prophase I, a prominent nucleolar region containing several dispersed nucleolar fragments was observed. In nuclei at diplotene-diakinesis, NORs were seen on an autosomal chromosome and on a sex chromosome (Figure 1a-c). 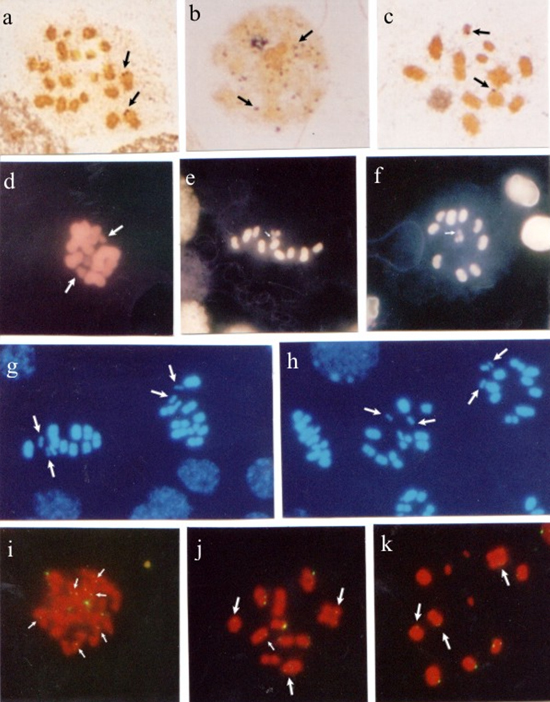
Figure 1. Panstrongylus megistus seminiferous tubules submitted to silver impregnation (a-c); double staining with fluorochromes CMA3/DA (d-f) and DAPI/DA (g,h), and fluorescent in situ hybridization (FISH) with Drosophila melanogaster 28S rDNA probe (i-k). Spermatogonial metaphases (a,d). Arrows indicate nucleolar organizer regions (NORs) in a, and CMA- chromosomes in d. Nuclei at meiotic prophase I (b,i). Arrows indicate silver-impregnated regions in b, and rDNA sites in i. Nuclei at diplotene-diakinesis (c,j,k). Arrows indicate NORs in c, and hybridization regions in j,k. First meiotic division metaphases (e,g,h). Arrows indicate a CMA- in e, and two DAPI- sex chromosomes in g,h. Second meiotic division metaphases (f), where the arrow indicates a CMA- sex chromosome. Bar = 10 µm. In testicular cells submitted to CMA3/DA staining, spermatogonial metaphases exhibited some CMA3- chromosomes. At first meiotic division metaphase (MI) and second meiotic division metaphase (MII), a CMA3- sex chromosome (Figure 1d-f), and two DAPI- sex chromosomes (Figure 1g,h) were observed. FISH with a 28S-12-kb rDNA probe stained several chromosomal regions in nuclei at meiotic prophase I. In nuclei at diplotene-diakinesis, hybridization with the rDNA probe was observed on some autosomal chromosomes (Figure 1 i-k). Rhodnius pallescensIn seminiferous tubule cells at MII, two sex chromosomes were silver-stained (Figure 2a,b). In the meiotic cells at MII, C banding showed evidence of two heterochromatic chromosomes, which were sometimes both sex chromosomes and in other cases a sex chromosome and the interstitial region of one of the autosomes (Figure 2c,d). 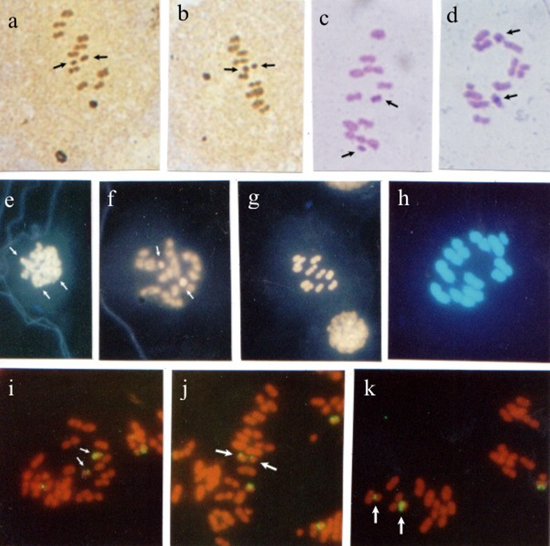 Figure 2. Rhodnius pallescens seminiferous tubules submitted to silver impregnation (a,b); C banding (c,d), double staining with fluorochromes CAM3/da (e-g) and dapi/da (h), and fluorescent in situ hybridization (FISH) with Drosophila melanogaster 28S rDNA probe (i-k). Spermatogonial metaphases (e); arrows indicate CMA+ chromosomes. Nuclei at diplotene-diakinesis (f), where arrows indicate two CMA+ regions. First (c,d,g,h,i,j,k) and second (a,b) meiotic division metaphases; arrows indicate NORs on sex chromosomes X and Y in a,b, and heterochromatic chromosomes and regions in c,d. In c, both sex chromosomes (X, Y) are heterochromatic, while in d one of the sex chromosomes (X) is heterochromatic, and one of the autosomes shows a heterochromatic block in the median region of the chromosome. In g,h, the chromosomes stained with fluorochromes CMA3/DA and DAPI/DA, respectively, showed no distinct regions. Hybridization signals with rDNA probe on the sex chromosomes (X, Y) were observed in i-k (arrows). Bar = 10 µm. In meiotic cells submitted to CMA3/da staining, spermatogonial metaphases showed some CMA3+ regions which could have been the sex chromosomes. At MI, the use of fluorochromes CMA3/DA and DAPI/DA did not yield chromosome differentiation (Figure 1e-h). FISH with the 28S-12-kb rDNA probe allowed the observation of hybridization signals on both sex chromosomes (X, Y). Triatoma infestansMeiotic cells impregnated with silver exhibited some silver-stained chromosomes in the nuclei at diplotene-diakinesis. At anaphase I, some chromosomal regions showed staining (Figure 3a-c). 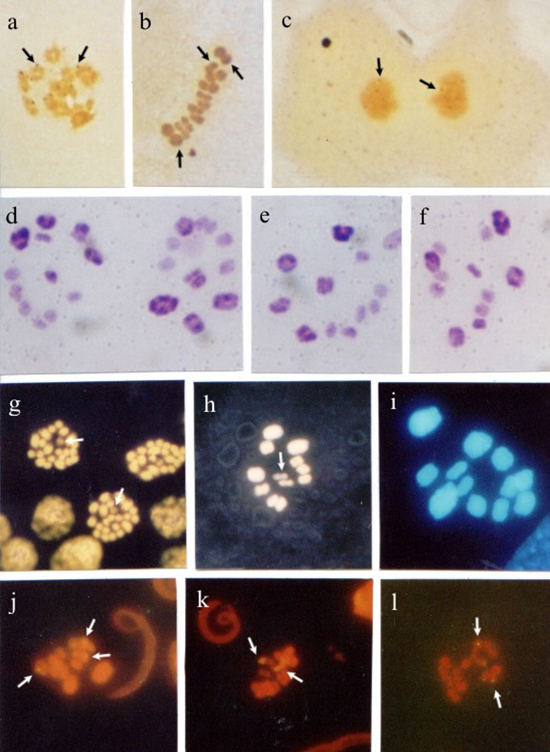 Figure 3. Triatoma infestans seminiferous tubules submitted to silver impregnation (a-c); C banding (d-f), double staining with fluorochromes CMA3/DA (g,h) and DAPI/DA (i), and fluorescent in situ hybridization (FISH) with Drosophila melanogaster 28S rDNA probe (j-l). Spermatogonial metaphases (g). Nuclei at diplotene-diakinesis (a,j). First (d,e,f,i) and second (h,k) meiotic division metaphases. Anaphases (c,l). Arrows indicate chromosomes with silver-impregnated regions (a-c). In d-f, four autosomal chromosomes and both sex chromosomes (X, Y) are heterochromatic. Arrows indicate CMA- chromosomes in g,h. In i, dapi/da staining showed no distinction among chromosomes. In j-l, arrows indicate regions hybridized by the probe. Bar = 10 µm. The use of C banding differentiated four autosomes at first division metaphase: one was completely heterochromatic; another had heterochromatic telomeric regions; another had two heterochromatic regions (one telomeric and the other interstitial), and one other had a heterochromatic interstitial region. Both sex chromosomes also showed heterochromatic interstitial regions (Figure 3d-f). In meiotic cells submitted to CMA3/DA staining, CMA3- chromosomes were seen in the spermatogonial metaphases, while a CMA3- sex chromosome was observed at MI. DAPI/DA did not discriminate chromosomes at MI (Figure 3g-i). FISH with a 28S rDNA probe showed some chromosomes with a hybridization signal in the nuclei at diplotene-diakinesis, as in MI and meiotic anaphases (Figure 3j-l). DISCUSSION Heterochromatin is considered to be responsible for creating new genes, maintaining chromosomes, recognizing chromosomes in the pairing of homologs and non-homolog chromosomes at meiosis, regulating recombination and segregation, and also, restoring telomeric stability after centric fission (John, 1988; Imai et al., 1988; Irick, 1994). In a variety of organisms, heterochromatin is completely eliminated from somatic cells. However, it is always retained in cells of the germinative strain. In meiotic cells, heterochromatin seems to be related to the formation of chiasmata, as crossing-over rarely occurs in heterochromatic regions (John and Miklos, 1979; Sumner, 1990). The characterization and identification of Heteropteran holocentric chromosomes is usually hindered by the fact that they are difficult to be morphologically differentiated and display a high chromosome condensation. In this study, however, both sex chromosomes (X, Y), or sometimes just one of them, along with several autosomes were observed to be heterochromatic in R. pallescens at MI. The analysis of T. infestans at MI demonstrated that four autosomal chromosomes (3 with telomeric blocks and 1 with an interstitial block) and both sex chromosomes (X, Y) (1 with a telomeric block and 1 with an interstitial block) are heterochromatic. These findings are not consistent with some classical reports, which describe a distinct pattern in T. infestans, where only three autosomal chromosomes (with telomeric chromosome blocks) and the Y sex chromosome are heterochromatic (Solari, 1979; Panzera et al., 1992). The difference in heterochromatin pattern found in this species could be related to the long-term maintenance of the colonies in laboratories, in specific crystallizers, which favors crossing-over. This is likely to have somehow influenced the heterochromatic pattern of the chromosomes in the specific case of the strain used in this study. Differences in heterochromatin amount and location on chromosomes of different species of the same genus and among individuals of the same species are very common. Thus, closely related species may differ in number of C+ bands, as well as in heterochromatin amount and location (Sumner, 1990; Clark and Wall, 1996). Panzera et al. (2000) analyzed several Triatoma species and suggested that the rate of chromosomal evolution is related to adaptation to different environments or to some properties intrinsic to the chromosome complement. These authors observed morphologic, ecologic and molecular differences among the chromatin forms of the species, indicating that the populations were undergoing differentiation processes that did not involve chromosome organization. Dujardin et al. (1999) supported this hypothesis by reporting that the differentiation that occurs among populations of a given species may lead to a speciation process that in Triatominae seems to be brief and, above all, induced by ecological factors. The hypothesis raised by these investigators supports the notion that the increase in heterochromatic blocks is due to the crossing-over caused by confining the T. infestans strain used in this study in crystallizers. Our results show that, when CMA3/DA and DAPI/DA were employed, the P. megistus species exhibited some CMA- chromosomes at spermatogonial metaphases, and two CMA3+/DAPI- sex chromosomes (X1X2Y) at meiotic metaphases (MI and MII), confirming the presence of regions rich in CG bases. These findings are consistent with the C banding study conducted by Tartarotti and Azeredo-Oliveira (1999) in P. megistus meiotic chromosomes. The species T. infestans studied herein showed some CMA3- chromosomes at spermatogonial metaphases, including one of the sex chromosome (X, Y) at MI, which indicated few CG regions. These findings support the results obtained by Mello and Recco-Pimentel (1987). DAPI banding failed to display heterochromatin regions. In R. pallescens, both spermatogonial metaphases and nuclei at meiotic prophase showed some chromosome regions rich in CG bases (CMA3+). Chromosomes at MI showed no heterochromatic regions when submitted to cma3/da and dapi/da as the chromosomes were homogeneously stained. In the species under study, the analyses of meiotic cells with the techniques of silver-impregnation and FISH with D. melanogaster 28S-12-kb rDNA probe revealed some correspondence between NORs detected by silver staining and the regions indicated by the rDNA probe. Some nonspecific staining was observed, as in P. megistus, but it did not affect data analysis. R. pallescens sex chromosomes (XY) were distinct from autosomes when submitted to silver impregnation and FISH, confirming that these chromosomes bear NORs. In P. megistus, the nuclei at early prophase I and at diakinesis exhibited corresponding staining when the techniques of silver impregnation and hybridization were used. In this species, NORs were observed on autosomal chromosomes at spermatogonial metaphase, and a sex chromosome, as well as an autosome bearing NORs were seen at diakinesis. In T. infestans, correspondence between silver staining and hybridization spots was demonstrated at diakinesis and at anaphase. In a study of four triatomine species of the genus Triatoma, the use of D. melanogaster 28S-12-kb rDNA probe revealed that in two of these species (T. tibiamaculata and T. protacta), some of the autosomes exhibited hybridization spots. In T. tibiamaculata, such spots were telomeric and found in only one bivalent. These spots seemed to coincide with CMA3+ regions. In T. platensis, a fluorescent spot seen in one of the sex chromosomes (probably the X chromosome) coincided with the silver-stained area. In T. vitticeps, hybridization was detected in two of the three sex chromosomes (Severi-Aguiar and Azeredo-Oliveira, 2005; Severi-Aguiar et al., 2006). The results obtained in the present study indicate that in triatomines, in general, the number and location of NORs are species-specific and, as in other animal groups, important chromosome markers that facilitate comparative studies for a better understanding of the evolutionary mechanisms of these important hematophagous insects. ACKNOWLEDGMENTS The authors are thankful to Dr. José M. Soares Barata, Director of the Insectary (Araraquara, SP), Department of Epidemiology, Faculty of Public Health (São Paulo, SP), and João Luís Molina Gil and João Maurício Nóbrega da Silva Filho, technicians of the insectary, for providing the insects studied. Prof. Klélia Aparecida de Carvalho and Prof. Dr. Shirlei M. Recco-Pimentel helped teach our group the FISH technique and provided the probe used. research supported by FAPESP (No. 00/06700-4) and CAPES. REFERENCES Clark MS and Wall WJ (1996). Chromosomes. The complex code. Chapman and Hall, London. De Vaio ES, Grucci B, Castagnino AM, et al. (1985). Meiotic differences between three triatomine species (Hemiptera- Reduviidae). Genetica 67: 185-191. Dujardin JP, Panzera P and Schofield CJ (1999). Triatominae as a model of morphological plasticity under ecological pressure. Mem. Inst. Oswaldo Cruz 94: 223-228. Fujiwara A, Abe S, Yamaha E, Yamazaki F, et al. (1998). Chromosomal localization and heterochromatin association of ribosomal RNA gene loci and silver-stained nucleolar organizer regions in salmonid fishes. Chromosome Res. 6: 463-471. Howell WM and Black DA (1980). Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36: 1014-1015. Hughes-Schrader S and Schrader F (1961). The kinetochore of the Hemiptera. Chromosoma 12: 327-350. Imai HT, Taylor RW, Crosland MW and Crozier RH (1988). Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn. J. Genet. 63: 159-185. Irick H (1994). A new function for heterochromatin. Chromosoma 103: 1-3. John B (1988). The biology of heterochromatin. In: Heterochromatin: molecular and structural aspects (Verma RS, ed.). Cambridge University Press, Cambridge, 301. John B and Miklos GL (1979). Functional aspects of satellite DNA and heterochromatin. Int. Rev. Cytol. 58: 1-114. Lourenço LB, Recco-Pimentel SM and Cardoso AJ (1998). Polymorphism of the nucleolus organizer regions (NORs) in Physalaemus petersi (Amphibia, Anura, Leptodactylidae) detected by silver staining and fluorescence in situ hybridization. Chromosome Res. 6: 621-628. Mello MLS and Recco-Pimentel SM (1987). Response to banding and Hoechst 33258 treatment in chromocentres of the malpighian tubule cells of Triatoma infestans. Cytobios 52: 175-184. Panzera F, Alvarez F, Sanches-Rufas J, Perez R, et al. (1992). C-heterochromatin polymorphism in holocentric chromosomes of Triatoma infestans (Hemiptera: Reduviidae). Genoma 35: 1068-1074. Panzera F, Pérez R, Nicolini P, Hornos S, et al. (2000). Chromosome homogeneity in populations of Triatoma brasiliensis Neiva 1911 (Hemiptera - Reduviidae - Triatominae). Cad. Saúde Pública 16: 83-88. Schmid M (1980). Chromosome banding in amphibia. IV. Differentiation of GC- and AT-rich chromosome regions in Anura. Chromosoma 77: 83-103. Severi-Aguiar GD and Azeredo-Oliveira MT (2005). Localization of rDNA sites in holocentric chromosomes of three species of triatomines (Heteroptera, Triatominae). Genet. Mol. Res. 4: 704-709. Severi-Aguiar GD, Lourenço LB, Bicudo HE and Azeredo-Oliveira MT (2006). Meiosis aspects and nucleolar activity in Triatoma vitticeps (Triatominae, Heteroptera). Genetica 126: 141-151. Solari AJ (1979). Autosomal synaptonemal complex and sex chromosomes without axes in Triatoma infestans (Reduviidae, Hemiptera). Chromosoma 72: 225-240. Sumner AT (1972). A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75: 304-306. Sumner AT (1990). Chromosome banding. Unwin Hyman, London. Tartarotti E and de Azeredo-Oliveira MT (1999). Heterochromatin patterns in triatomines of the genus Panstrongylus. Cytobios 99: 113-122. Viegas-Péquignot E (1992). In situ hybridization to chromosomes with biotinylated probes. In: In situ hybridization: a practical approach (Willman D, ed.). Oxford University Press, IRL Press, Oxon, England, 137-158. |
|