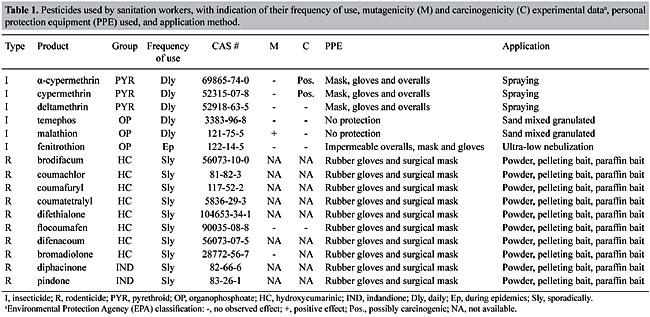
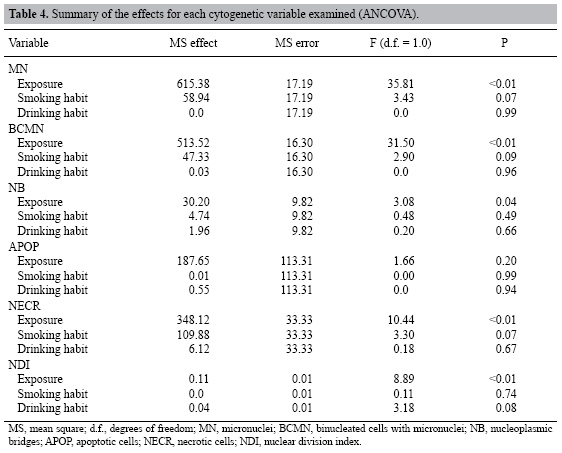
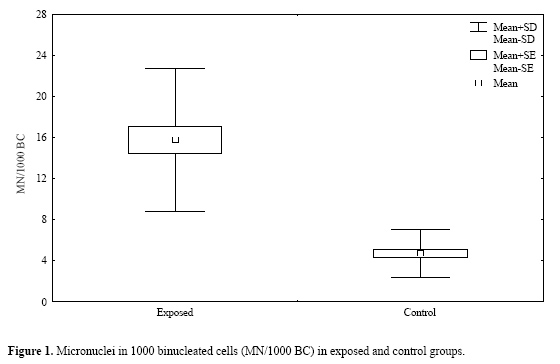
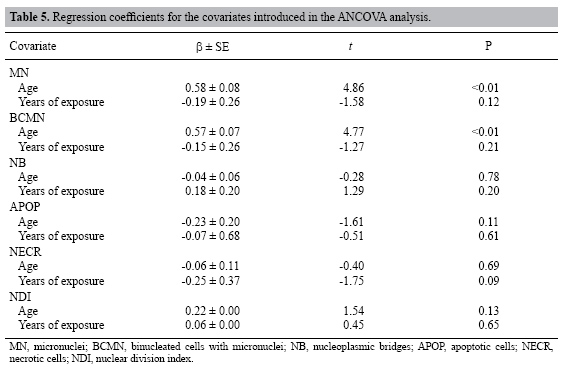
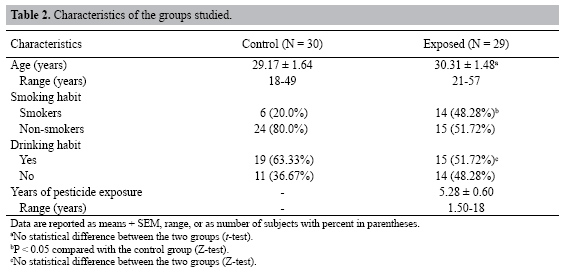
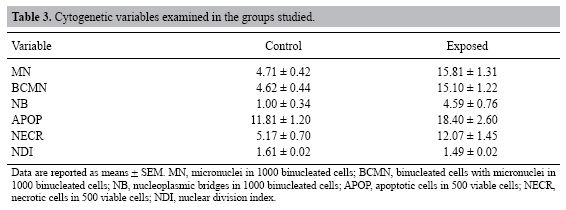
| Study of the cytogenetic effects of occupational exposure to pesticides on sanitation workers in Belo Horizonte, Brazil F.S.G. Kehdy1, E.M.M. Cerqueira3, M.B. Bonjardim1, R.M. Camelo2 and M.C.L. Castro1 1Departamento de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil 2Departamento de Farmacologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil 3Universidade Estadual de Feira de Santana, Feira de Santana, BA, Brasil Corresponding author: F.S.G. Kehdy E-mail: [email protected] Genet. Mol. Res. 6 (3): 581-593 (2007) Received April 16, 2007 Accepted August 30, 2007 Published September 30, 2007 ABSTRACT. Sanitation workers handling pesticides in the control of disease vectors constitute an occupationally exposed population to genotoxic substances. The aim of the present study was to investigate the relation between the occupational exposure to various pesticides and the presence of cytogenetic damage. Fifty-nine men were selected (29 sanitation workers and 30 control individuals) with ages varying between 18-57 years who lived and worked in the same area in Belo Horizonte (Brazil). The following parameters were determined for all individuals using the cytokinesis-block micronucleus (MN) assay in peripheral blood lymphocytes: MN/1000 binucleated cells (BC), BC with MN (BCMN)/1000 BC, nucleoplasmic bridges (NB)/1000 BC, apoptotic and necrotic cells/500 cells and nuclear division index. The analysis of covariance showed significantly higher (p < 0.05) mean frequencies of MN (15.81 ± 1.31 vs 4.71 ± 0.42), BCMN (15.10 ± 1.22 vs 4.62 ± 0.44), NB (4.59 ± 0.76 vs 1.00 ± 0.34), and necrotic cells (12.07 ± 1.45 vs 5.17 ± 0.70) in the exposed group when compared to the control group. There was no significant difference in the apoptotic cell frequency between the two groups, while the nuclear division index was significantly lower (1.49 ± 0.02 vs 1.61 ± 0.02) in the control group. Neither the time of exposure nor the smoking or alcohol drinking habit influenced the cytogenetic parameters examined. According to these results, occupational exposure to pesticides induced genotoxic and cytotoxic effects in sanitation workers. Key words: Pesticide exposure, Biomonitoring, Micronucleus test, Sanitation workers INTRODUCTION Pesticides are a group of natural or synthetic chemical substances designated to combat plagues that generally attack, harm or transmit illness to living organisms including humans (Kolaczinski and Curtis, 2004). Although they may be selective against specific organisms (such as bacteria, fungi, undergrowth, and rodents), most of them do not have an absolute selectivity, becoming a potential risk to human health (Bolognesi, 2003). Many studies showed an association between the exposure to pesticides and the increase in the incidence of some cancers including non-Hodgkin’s lymphoma (Hardell and Eriksson, 1999; Zheng et al., 2001), multiple myeloma (Khuder and Mutgi, 1997), sarcomas (Blair et al., 1983; Kogevinas et al., 1995), and pancreatic (Ji et al., 2001) and bladder cancers (Shukla et al., 2001). The genotoxic effects of a pesticide are primary factors for carcinogenesis, and thus, genotoxicologic biomonitoring will become useful in human populations exposed to them (Bolognesi, 2003). Meanwhile, the results of this kind of biomonitoring obtained until now are ambiguous (Scarpato et al., 1996a; Venegas et al., 1998; Gomez-Arroyo et al., 2000; Gregio D’Arce and Colus, 2000; Lucero et al., 2000), probably due to different conditions of the populations studied, the specific genotoxic effect of the different pesticides employed, and to interindividual variability (Bolognesi, 2003). In tropical countries, illnesses such as dengue, malaria, yellow fever, leishmaniasis, and leptospirosis are public health problems. For such diseases, environmental conditions can allow the development and proliferation of insects or other vectors (Neves and Filippis, 2003). The struggle to eliminate these animals is a task performed by the government at different levels, with the promotion of several control measures, including the application of pesticides in areas with high concentrations of cases. The sanitation workers engaged in the application of pesticides are occupationally exposed to potential genotoxic substances, making them a cancer risk population (Bolognesi, 2003). This study aimed to evaluate the genotoxic effects of occupational exposure to pesticides in a sample of sanitation workers of Belo Horizonte, State of Minas Gerais, Brazil. The micronucleus (MN) lymphocyte culture test was employed. It is a cytogenetic method that measures breaks (clastogenic effect) and chromosome losses (aneugenic effect) in binucleated cells (Fenech, 1993). Other cytogenetic parameters, namely nucleoplasmic bridges (NB), apoptotic (APOP) and necrotic (NECR) cells, and nuclear division index (NDI), were also determined (Fenech, 2000). The factors age and smoking and alcohol drinking habits were taken into account because they may influence the expression of the cytogenetic parameters examined (Fenech, 1998). MATERIAL AND METHODS Subjects Fifty-nine males between 18 and 57 years old agreed to take part in the research, between August and October of 2004. All the participants lived and worked in Belo Horizonte (Brazil) and they did not show any serious morbidity at the time of the sample collection. The exposed group included 29 sanitation workers of the City Hall of Belo Horizonte who were occupationally exposed to various pesticides. The control group was selected from population that worked next to the exposed individuals and included 30 volunteers who had never been occupationally exposed to pesticides. All the individuals answered a questionnaire, supplying information related to age, smoking and alcohol drinking habits and, in the case of the exposed group, the duration and the frequency of exposure and the personal protection equipment (PPE) employed. People were considered smokers when they usually smoked 15 or more cigarettes per day for at least one year, and people who often drank any alcoholic beverage two or more times per week were considered alcohol drinkers. The project was approved by the Committee on Ethics and Research of the Federal University of Minas Gerais (ETIC 373/04) and all the subjects signed an informed consent form. Blood sample collection and cell culture Ten milliliters of peripheral blood was obtained from each subject by venipuncture using heparinized vacutainers. Mononuclear cells were separated in a Histopaque® density gradient (Sigma) and added (106 cells/mL) to an RPMI 1640 (Gibco) medium supplemented with 10% (v/v) fetal bovine serum (Gibco), 2 mmol/L glutamine (Gibco), antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin and 25 µg/mL amphotericin B; Gibco) and 2% (v/v) phytohemagglutinin A (Gibco). The cultures were maintained at 37ºC in a 5% CO2 incubator. After 44 h, cytochalasin B was added to the culture (6 µg/mL; Sigma) (Fenech, 2000). At the end of incubation (72 h), the cells were centrifuged at room temperature and gently suspended in a cold 70% (v/v) methanol:acetic acid (3:1) fixative solution. This procedure was performed twice. The cell suspension was dropped onto three previously labeled clean slides. The slides were air-dried and stained with Giemsa solution (4%, v/v; Gibco) in Dulbecco’s phosphate-buffered saline, pH 7.1 (Gibco) for 15 min. Slide analysis The slides were analyzed blindly using a light microscope with a 1000X lens. For each individual, 500 lymphocytes were analyzed in order to determine the APOP cell frequency, the NECR cell frequency and the number of cells with one to four nuclei was computed to calculate the NDI. This index is determined by the formula: NDI = (MC + 2x BC + 3x TC + 4x QC) / total viable cells, where MC-QC represents the number of cells with one to four nuclei, respectively. The frequencies of MN, binucleated (BC) cells with MN (BCMN) and the NB were determined by counting 1000 BC viable cells with preserved cytoplasm. BC, MN, NB, APOP, and NECR were determined in agreement with previously described criteria (Fenech et al., 2003). Statistical analysis In order to evaluate the possible differences between the control group and the exposed one in relation to age, the unpaired Student t-test was performed. In relation to smoking and alcohol drinking habits, the possible differences between the two groups were analyzed by the Z-test for two independent proportions. The effects of exposure and smoking and alcohol drinking habits on the cytogenetic variables (MN, BCMN, NB, APOP, and NECR) and on the NDI were evaluated by the analysis of covariance (ANCOVA) including age and time of exposure as covariates. The statistical analyses were performed with the STATISTICA software (5.0 for Windows). Differences were considered statistically significant when p values were less than 0.05. RESULTS The sanitation workers included in this study were exposed to several pesticides. As shown in Table 1, some of these pesticides are mutagenic and/or possibly carcinogenic composites and belong to the organophosphate and pyrethroid insecticides and hydroxycoumarinic rodenticides. The application of the composites was performed by spraying (pyrethroids), powder, pelleting bait, paraffin bait (hydroxycumarin and indandione), and ultra-low volume pulverization or sand-mixed granulated (organophosphate). The composites were applied separately and the PPE used was specific for each composite, except for malathion and temephos whose application was performed without PPE. The majority of the pesticides are used sporadically. All the exposed individuals worked for 40 h a week. The main characteristics of the population are described in Table 2. Age and alcohol drinking habit were similar in the two groups. The exposed group had a larger number of smokers (p < 0.05, Z-test). The average pesticide exposure time of the workers was 5.28 + 0.60 years. Table 3 summarizes the mean values of the cytogenetic variables and NDI examined in the groups. The ANCOVA results and the regression coefficients are presented in Tables 4 and 5, respectively. The exposed group showed MN frequencies, BCMN, NB, and NECR significantly higher than the control group (p < 0.01) (Table 4). Figure 1 shows the MN frequencies in both groups. Although the difference in APOP frequency was not significant, it was higher in the exposed group (Table 4). The exposed group had significantly lower NDI values (p < 0.01) (Table 4). The regression coefficients (Table 5) indicated that, from the covariates introduced in the analysis, only the age of the individuals had a significant influence on the MN and BCMN frequencies (P < 0.01). The pesticide exposure time did not show an influence on the parameters examined (Table 5). Neither the smoking habit nor alcohol drinking influenced the cytogenetic variables studied (Table 4).
DISCUSSION With the increase in global population, new areas are being rough-hewed, and the humans are more exposed to illnesses transmitted by wild vectors (Neves and Filippis, 2003). The control of the increase of infected, transmitting organisms has become a central issue in sanitation surveillance managed by governments. The use of pesticides has become routine, mainly in underdeveloped countries, but the genotoxic potential of these substances is not yet well established (Bolognesi, 2003). Most of the population that lives in the affected areas and the sanitation workers responsible for the application are at risk for cytotoxicity and genotoxicity.
The aim of this study was to determine if occupational exposure to pesticides could cause cytogenetic damage compared to a control group that has never been exposed to these chemicals. Fifty-nine males (29 occupationally exposed and 30 control subjects) were included in the study. The age of the subjects and alcohol drinking habits were similar in the two groups, but there were a larger number of smokers in the exposed group. In spite of that, this difference did not influence the results, since analysis of covariance showed that the smoking habit did not have a significant effect on the cytogenetic parameters examined. To evaluate the genetic damage that has taken place in these subjects, the cytokinesis-block micronucleus assay in human lymphocyte cultures (CBMN assay) was used (Fenech and Morley, 1985; Fenech, 1997). Through this assay, clastogenic and aneugenic effects are detected since the MN originate from chromosomal fragments or from an entire chromosome not included in the main nucleus of the descendent cells during cell division (Fenech, 1997; Kirsch-Volders et al., 1997). Recently, the inclusion of other cytogenetic parameters in the CBMN assay has been proposed (Thomas et al., 2003). These parameters are the presence of nucleoplasmic bridges (indicators of chromosomal rearrangements), apoptotic and necrotic cells (indicators of cell viability), and cell division index (Fenech et al., 1999; Kirsch-Volders and Fenech, 2001). Because this test offers simultaneous information on DNA damage and cytotoxic/cytostatic effects caused by possibly aggressive agents, nowadays it is a simple and important tool for the monitoring of human populations (Kirsch-Volders and Fenech, 2001).
In this study, the group exposed to pesticides showed a significantly higher frequency of chromosome damage (MN, BCMN and NB) when compared to the control group. Some studies have also shown a positive association between genotoxicity and occupational exposure to pesticides (De Ferrari et al., 1991; Carbonell et al., 1993; Kourakis et al., 1992, 1996; Garaj-Vrhovak and Zeljezic, 1999; Falck et al., 1999; Gomez-Arroyo et al., 2000; Shaham et al., 2001; Zeljezic and Garaj-Vrhovac, 2001), but other studies did not conclude the same (Gomez-Arroyo et al., 1992; Scarpato et al., 1996a,b; Pasquini et al., 1996; Davies et al., 1998; Gregio D’ Arce and Colus, 2000; Lucero et al., 2000; Pastor et al., 2001a,b). Such disagreement may be explained by either different exposure conditions (protection measure used and specific genotoxic potential of the substances used) or by demographic factors, individual habits and associated genetic features (Fenech, 1998; Bolognesi, 2003). Therefore, each biomonitoring study is unique and, in order to estimate the effects of an occupational exposure, each population should be studied separately and the results should not be generalized. The higher occurrence of chromosomal damage in the exposed group can be explained by the genotoxic potential of the pesticides to which they were exposed. The pyrethroid group is the most frequently used in solution through spraying. When applying it the workers make use of PPE. Of the pyrethroids used by the workers, the U.S. Environmental Protection Agency (EPA) has classified only cypermethrin as a possible carcinogen, while the other pyrethroids do not have mutagenic and/or carcinogenic activity. This reduces the possibility of the remaining pyrethroids (except cypermethrin) being responsible for the damage detected. The organophosphate group was applied daily in the solid form without using PPE. In agreement with the EPA classification, malathion showed mutagenic activity in the experimental system, but carcinogenic activity was not observed. Other substances (hydroxycoumarin and indandione) were sporadically applied with the use of PPE. So far, most of them have not been tested for their mutagenic and carcinogenic effects, and so it is not possible to say if the exposure to these substances could be considered safe. This suggests that malathion (organophosphate) and cypermethrin (pyrethroid) should be the main pesticides responsible for the chromosome damage found in the exposed workers. However, it is important to note that an exposure to a great number of different compounds makes it difficult to know which agent could be responsible for the observed cytogenetic damages. Another aspect that could contribute to the positive association between the observed cytogenetic damage and pesticide exposure is that most of the biomonitoring studies in occupationally exposed populations are conducted with individuals that use a mix of several chemical compounds (Lucero et al., 2000; Bolognesi et al., 2002; Pastor et al., 2001a,b, 2002, 2003). In this study, the pesticides were applied separately and probably in higher concentrations than those found in mixtures, and these concentrations may show a higher genotoxic potential. In spite of the use of protective equipment in the application of most products, an induction of chromosome damage caused by pesticide exposure was still observed. These findings can be explained by the ineffectiveness or inappropriate use of the protective measures. Another explanation could be the fact that malathion organophosphate may be the main substance responsible for the chromosome damage found, because it was applied without PPE. Cytotoxicity was higher in the sanitation workers than in the control group, since the frequency of necrotic cells was significantly higher in the exposed group. One possibility is the existence of two groups of substances: a genotoxic group that causes chromosome damage without killing the cells and a cytotoxic group that causes cell necrosis. The fact is that the surviving cells are perpetuating important mutations demonstrated by the increased frequencies of MN, BCMN and NB. There was no significant difference in APOP frequency between the exposed and the control groups. This suggests that the DNA damage caused by the exposure was not sufficient to cause apoptosis, but did cause MN and NB (Fenech et al., 1999). The reduction in the NDI found in the exposed group, according to other studies (Pastor et al., 2001b, 2002), corroborates the hypothesis that cells with DNA damage delay the cell cycle in order to repair the damage and avoid the fixation of mutations during replication (Kirsch-Volders and Fenech, 2001). However, the fact that MN, BCMN and NB frequencies have been higher in the exposed group shows that the repair may not be efficient to correct the induced mutations caused by exposure. Another hypothesis is that the chemicals have cytotoxic properties that affect the cell proliferation kinetics (Rupa et al., 1991; Pasquini et al., 1996). Of the variables studied that could influence the cytogenetic parameters determined, only age was positively related to MN and BCMN frequencies. These results agree with many other studies that have shown an increase in spontaneous MN frequency with age (Fenech and Morley, 1986; Ramsey et al., 1995; Fenech, 1998; Barale et al., 1998). This effect has been attributed to an increase in aneuploidy mainly of the X and Y chromosomes (Norppa and Falck, 2003). An association between the cytogenetic parameters and the time of exposure of the workers to the pesticides was not observed. This observation differs from the results found by other authors. Bolognesi et al. (2002) found a positive relation between MN incidence and the duration of pesticide exposure when individuals were exposed for more than 10 years, suggesting that chromosome damage is accumulated during continuous exposure to pesticides. The lack of association between the cytogenetic parameters determined and time of exposure in the present study could be explained by the slight variation in the exposure time. In this study, exposure time was from 1.5 to 18 years, and only one individual was exposed for more than 10 years. Smoking habit did not influence the cytogenetic parameters examined. In the biomonitoring studies of populations occupationally exposed to genotoxic agents, the smoking habit influence on MN frequency is controversial. Few studies have shown an association between these variables (da Cruz et al., 1994; Di Giorgio et al., 1994), while most of the studies of this nature have not found any association at all (Lucero et al., 2000; Pastor et al., 2001a,b, 2002; Bolognesi et al., 1993, 2002). A possible explanation is that the damage caused by tobacco could kill the cells in culture or delay the cell cycle, making it impossible to carry out the MN test (Bonassi et al., 2003). However, there was no influence of smoking habit on APOP/NECR frequencies nor on NDI. Another possibility is that the small number of smokers in the control group (6 individuals) constitutes a non-representative sample. Like the smoking habit, the alcohol drinking habit did not influence the parameters examined. Data in the literature showed positive, preventive or no effects (da Cruz et al., 1994; Surrallés et al., 1997; Barale et al., 1998; Castelli et al., 1999) of alcohol drinking habit on MN frequency. Normally, these effects occur as a consequence of the relation between alcohol drinking and other variables, such as age (Pastor et al., 2001a). In conclusion, occupational exposure to pesticides by sanitation workers in Belo Horizonte showed genotoxic and cytotoxic effects, demonstrated by higher frequencies of MN, BCMN, NB, and NECR cells when compared with the control group. The reduced NDI in the exposed group suggests a possible adaptive response to the chronic exposure to pesticides. The age factor showed a direct relation with MN frequency, while the smoking and drinking habits did not influence the cytogenetic parameters determined. These results provide evidence of the genetic hazard related to occupational pesticide exposure and, therefore, of the need for educational programs to encourage the correct use of PPE and/or implementation of new protective measures for sanitation workers. ACKNOWLEDGMENTS We are very thankful to the volunteers who participated in the study, to the Laboratory of Antitumor Substances (Prof. Dr. Miriam T.P. Lopes) and to Marlene de Miranda for collecting blood samples. Research supported by CAPES. REFERENCES Barale R, Chelotti L, Davini T, Del Ry S, et al. (1998). Sister chromatid exchange and micronucleus frequency in human lymphocytes of 1,650 subjects in an Italian population: II. Contribution of sex, age, and lifestyle. Environ. Mol. Mutagen. 31: 228-242. Blair A, Grauman DJ, Lubin JH and Fraumeni JF Jr (1983). Lung cancer and other causes of death among licensed pesticide applicators. J. Natl. Cancer Inst. 71: 31-37. Bolognesi C (2003). Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat. Res. 543: 251-272. Bolognesi C, Parrini M, Bonassi S, Ianello G, et al. (1993). Cytogenetic analysis of a human population occupationally exposed to pesticides. Mutat. Res. 285: 239-249. Bolognesi C, Perrone E and Landini E (2002). Micronucleus monitoring of a floriculturist population from western Liguria, Italy. Mutagenesis 17: 391-397. Bonassi S, Neri M, Lando C, Ceppi M, et al. (2003). Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat. Res. 543: 155-166. Carbonell E, Xamena N, Creus A and Marcos R (1993). Cytogenetic biomonitoring in a Spanish group of agricultural workers exposed to pesticides. Mutagenesis 8: 511-517. Castelli E, Hrelia P, Maffei F, Fimognari C, et al. (1999). Indicators of genetic damage in alcoholics: reversibility after alcohol abstinence. Hepatogastroenterology 46: 1664-1668. da Cruz AD, McArthur AG, Silva CC, Curado MP, et al. (1994). Human micronucleus counts are correlated with age, smoking, and cesium-137 dose in the Goiânia (Brazil) radiological accident. Mutat. Res. 313: 57-68. Davies HW, Kennedy SM, Teschke K, Jenny P, et al. (1998). Cytogenetic analysis of South Asian berry pickers in British Columbia using the micronucleus assay in peripheral lymphocytes. Mutat. Res. 416: 101-113. De Ferrari M, Artuso M, Bonassi S, Bonatti S, et al. (1991). Cytogenetic biomonitoring of an Italian population exposed to pesticides: chromosome aberration and sister-chromatid exchange analysis in peripheral blood lymphocytes. Mutat. Res. 260: 105-113. Di Giorgio C, De Méo MP, Laget M, Guiraud H, et al. (1994). The micronucleus assay in human lymphocytes: screening for inter-individual variability and application to biomonitoring. Carcinogenesis 15: 313-317. Falck GC, Hirvonen A, Scarpato R, Saarikoski ST, et al. (1999). Micronuclei in blood lymphocytes and genetic polymorphism for GSTM1, GSTT1 and NAT2 in pesticide-exposed greenhouse workers. Mutat. Res. 441: 225-237. Fenech M (1993). The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat. Res. 285: 35-44. Fenech M (1997). The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 392: 11-18. Fenech M (1998). Important variables that influence base-line micronucleus frequency in cytokinesis-blocked lymphocytes - a biomarker for DNA damage in human populations. Mutat. Res. 404: 155-165. Fenech M (2000). The in vitro micronucleus technique. Mutat. Res. 455: 81-95. Fenech M and Morley AA (1985). Measurement of micronuclei in lymphocytes. Mutat. Res. 147: 29-36. Fenech M and Morley AA (1986). Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutat. Res. 161: 193-198. Fenech M, Crott J, Turner J and Brown S (1999). Necrosis, apoptosis, cytostasis and DNA damage in human lymphocytes measured simultaneously within the cytokinesis-block micronucleus assay: description of the method and results for hydrogen peroxide. Mutagenesis 14: 605-612. Fenech M, Chang WP, Kirsch-Volders M, Holland N, et al. (2003). HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 534: 65-75. Garaj-Vrhovak V and Zeljezic D (1999). Chromosomal aberrations and frequency of micronuclei in workers employed in pesticide production. Biologia 54: 707-712. Gomez-Arroyo S, Noriega-Aldana N, Osorio A, Galicia F, et al. (1992). Sister-chromatid exchange analysis in a rural population of Mexico exposed to pesticides. Mutat. Res. 281: 173-179. Gomez-Arroyo S, Diaz-Sanchez Y, Meneses-Perez MA, Villalobos-Pietrini R, et al. (2000). Cytogenetic biomonitoring in a Mexican floriculture worker group exposed to pesticides. Mutat. Res. 466: 117-124. Gregio D’Arce LP and Colus IM (2000). Cytogenetic and molecular biomonitoring of agricultural workers exposed to pesticides in Brazil. Teratog. Carcinog. Mutagen. 20: 161-170. Hardell L and Eriksson M (1999). A case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer 85: 1353-1360. Ji BT, Silverman DT, Stewart PA, Blair A, et al. (2001). Occupational exposure to pesticides and pancreatic cancer. Am. J. Ind. Med. 39: 92-99. Khuder SA and Mutgi AB (1997). Meta-analyses of multiple myeloma and farming. Am. J. Ind. Med. 32: 510-516. Kirsch-Volders M and Fenech M (2001). Inclusion of micronuclei in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis block micronucleus assay for biomonitoring purposes. Mutagenesis 16: 51-58. Kirsch-Volders M, Elhajouji A, Cundari E and van Hummelen P (1997). The in vitro micronucleus test: a multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat. Res. 392: 19-30. Kogevinas M, Kauppinen T, Winkelmann R, Becher H, et al. (1995). Soft tissue sarcoma and non-Hodgkin’s lymphoma in workers exposed to phenoxy herbicides, chlorophenols, and dioxins: two nested case-control studies. Epidemiology 6: 396-402. Kolaczinski JH and Curtis CF (2004). Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem. Toxicol. 42: 697-706. Kourakis A, Mouratidou M, Kokkinos G, Barbouti A, et al. (1992). Frequencies of chromosomal aberrations in pesticide sprayers working in plastic green houses. Mutat. Res. 279: 145-148. Kourakis A, Mouratidou M, Barbouti A and Dimikiotou M (1996). Cytogenetic effects of occupational exposure in the peripheral blood lymphocytes of pesticide sprayers. Carcinogenesis 17: 99-101. Lucero L, Pastor S, Suárez S, Durban R, et al. (2000). Cytogenetic biomonitoring of Spanish greenhouse workers exposed to pesticides: micronuclei analysis in peripheral blood lymphocytes and buccal epithelial cells. Mutat. Res. 464: 255-262. Neves DP and Filippis T (2003). Parasitologia Básica. Ed. Coopemed, Belo Horizonte. Norppa H and Falck GC (2003). What do human micronuclei contain? Mutagenesis 18: 221-233. Pasquini R, Scassellati-Sforzolini G, Angeli G, Fatigoni C, et al. (1996). Cytogenetic biomonitoring of pesticide-exposed farmers in central Italy. J. Environ. Pathol. Toxicol. Oncol. 15: 29-39. Pastor S, Gutierrez S, Creus A, Cebulska-Wasilewska A, et al. (2001a). Micronuclei in peripheral blood lymphocytes and buccal epithelial cells of Polish farmers exposed to pesticides. Mutat. Res. 495: 147-156. Pastor S, Gutierrez S, Creus A, Xamena N, et al. (2001b). Cytogenetic analysis of Greek farmers using the micronucleus assay in peripheral lymphocytes and buccal cells. Mutagenesis 16: 539-545. Pastor S, Lucero L, Gutiérrez S, Durbán R, et al. (2002). A follow-up study on micronucleus frequency in Spanish agricultural workers exposed to pesticides. Mutagenesis 17: 79-82. Pastor S, Creus A, Parrón T, Cebulska-Wasilewska A, et al. (2003). Biomonitoring of four European populations occupationally exposed to pesticides: use of micronuclei as biomarkers. Mutagenesis 18: 249-258. Ramsey MJ, Moore DH, Briner JF, Lee DA, et al. (1995). The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat. Res. 338: 95-106. Rupa DS, Reddy PP and Reddi OS (1991). Clastogenic effect of pesticides in peripheral lymphocytes of cotton-field workers. Mutat. Res. 261: 177-180. Scarpato R, Migliore L, Angotzi G, Fedi A, et al. (1996a). Cytogenetic monitoring of a group of Italian floriculturists: no evidence of DNA damage related to pesticide exposure. Mutat. Res. 367: 73-82. Scarpato R, Migliore L, Hirvonen A, Falck G, et al. (1996b). Cytogenetic monitoring of occupational exposure to pesticides: characterization of GSTM1, GSTT1, and NAT2 genotypes. Environ. Mol. Mutagen. 27: 263-269. Shaham J, Kaufman Z, Gurvich R and Levi Z (2001). Frequency of sister-chromatid exchange among greenhouse farmers exposed to pesticides. Mutat. Res. 491: 71-80. Shukla VK, Rastogi AN, Adukia TK, Raizada RB, et al. (2001). Organochlorine pesticides in carcinoma of the gallbladder: a case-control study. Eur. J. Cancer Prev. 10: 153-156. Surrallés J, Autio K, Nylund L, Jarventaus H, et al. (1997). Molecular cytogenetic analysis of buccal cells and lymphocytes from benzene-exposed workers. Carcinogenesis 18: 817-823. Thomas P, Umegaki K and Fenech M (2003). Nucleoplasmic bridges are a sensitive measure of chromosome rearrangement in the cytokinesis-block micronucleus assay. Mutagenesis 18: 187-194. United States Environmental Protection Agency (EPA). EXTOXNET Pesticide Information Profiles. Available at http://extoxnet.orst.edu/pips/ghindex.html. Accessed February 2005. Venegas W, Zapata I, Carbonell E and Marcos R (1998). Micronuclei analysis in lymphocytes of pesticide sprayers from Concepcion, Chile. Teratog. Carcinog. Mutagen. 18: 123-129. Zeljezic D and Garaj-Vrhovac V (2001). Chromosomal aberration and single cell gel electrophoresis (Comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis 16: 359-363. Zheng T, Zahm SH, Cantor KP, Weisenburger DD, et al. (2001). Agricultural exposure to carbamate pesticides and risk of non-Hodgkin lymphoma. J. Occup. Environ. Med. 43: 641-649. |
|