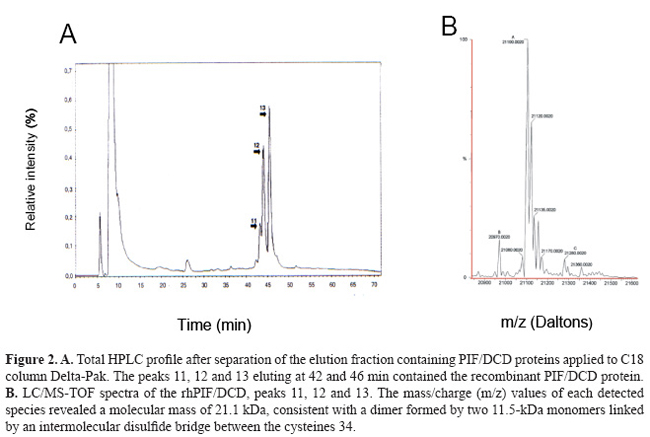
Prediction and biochemical characterization of intrinsic disorder in the structure of proteolysis-inducing factor/dermcidin G. Majczak1*, S. Lilla1*, M. Garay-Malpartida1, J. Markovic1, F.J. Medrano2, G. de Nucci1 and J.E. Belizário1 Genet. Mol. Res. 6 (4): 1000 - 1011 (2007) ABSTRACT. Proteolysis-inducing factor/dermcidin (PIF/DCD) is a novel human gene, located on chromosome 12, locus 12q13.1, that encodes a secreted 110-amino acid protein. Two transcripts for the protein have been identified in normal skin, breast, placenta and brain, and in various primary and metastatic tumor cells. The putative native-state structure of PIF/DCD has not been resolved. Here, we describe some biochemical features of the soluble recombinant 11-kDa protein produced in Escherichia coli. The native 11-kDa polypeptide displayed an anomalous mobility on 1% SDS-PAGE under reduced conditions and appeared as a single ~16-kDa band. Under nonreduced conditions, we detected by mass spectrometry, the presence of multiple peaks corresponding to m/z values of 21 kDa, which we confirmed as a dimeric form with a disulfide bridge between cysteine 34 of each 11-kDa monomer. The native protein exhibited an unusually high susceptibility to proteolytic attack by trypsin, and up to 13 peptides derived from its C-terminus were produced after 5 min of incubation. The secondary structure analysis of PIF/DCD native protein in aqueous solution, by circular dichroism spectroscopy, revealed regions with non-well-defined secondary structure but that acquired α-helix and β-sheet secondary structures in the presence of TFE/water mixtures and micellar and non-micellar SDS molecules. By using PONDR®, DisEMBL™, DisProt, and GlobPlot™ computational predictors, we identified a long disorder region at the N-terminus of PIF/DCD amino acid sequence. This segment (from 19-50 residues) is critical for some of its biological activities, including neuron survival. This result is coherent with successive failure of crystallization of the protein. Taken together, these data suggest that the disorder and order transition may be relevant for some biological functions of PIF/DCD. Key words: Antimicrobial peptides, Intrinsically unstructured proteins, Circular dichroism INTRODUCTION Proteolysis-inducing factor/dermcidin (PIF/DCD) is a novel human gene, located on chromosome 12, locus 12q13.1, whose transcripts have been identified in normal skin, mammary and brain tissues, and various cancer cells derived from primary and metastastic tumors (Schittek et al., 2001; Cunningham et al., 2002; Porter al., 2003; Wang et al., 2003; Deans et al., 2006). Previously, we identified that the levels of PIF/DCD mRNA expression were higher in some breast carcinoma cell lines when compared to normal breast, testis and placenta (Markovic, 2003). There has been considerable evidence from many studies that this gene is overexpressed in various cancer cells and likely plays a role in carcinogenesis and metabolic dysfunctions in the host (Porter et al., 2003; Wang et al., 2003; Minami et al., 2004; Lowrie et al., 2006). In addition, it has been demonstrated that the 11-kDa polypeptide is processed into different proteolytic peptides and that a 47-amino acid fragment derived from its C-terminal region, and 30- and 20-amino acid fragments derived from its N-terminal region, may function as either antibiotic peptides in the skin (Schittek et al., 2001), diffusible survival evasion peptides in the brain (Cunningham et al., 1998, 2002) or cancer cachectic factor in patients with pancreatic, breast, lung and ovarian cancer, and malignant melanoma (Todorov et al., 1996; Wang et al., 2003), respectively. We have undertaken various biochemical and biological experiments to define the amino acids and structures in PIF/DCD protein that may be critical for its putative native-state structure and multiple biological functions. First, we cloned the full-length cDNA of human PIF/DCD and purified recombinant forms of protein produced in Escherichia coli (Garay-Malpartida, 2004). Initial studies showed an unexpected feature of the protein with respect to its mobility on 15% polyacrylamide gels containing 1% SDS, under reduced conditions. The native protein exhibited a molecular mass estimated at 16 kDa, while the expected size was 11 kDa. In addition, our studies on limited proteolysis in the presence of trypsin revealed that the PIF/DCD protein has a high proteolytic susceptibility. These features are similar to proteins known as natively intrinsically unstructured proteins (Tompa, 2002). Polypeptides in this class exhibit an absence of globularity, low compactness, absence of secondary structure, and high flexibility, which are characterized experimentally by missing regions of electron density in crystal structures (Tompa, 2002). The availability of curated databases and development of computational tools have opened new perspectives and increased the success rate of assigning the amino acid residues involved in secondary structure formation such as α-helices, β-sheets, β-strands, and loops/coils in the proteins that lack three-dimensional structures (Iakoucheva and Dunker, 2003). Thus, we evaluated order and disorder in the PIF/DCD protein sequence using in silico web site programs. In addition, we carried out circular dichroism spectroscopy with PIF/DCD in aqueous solution and in the presence of solvents that revealed secondary structure contents and chemical properties for the protein, which may provide new clues to its molecular mechanism of action and biological activities. MATERIAL AND METHODS Protein expression and purification Full-length PIF/DCD cDNA (DKFZp313H1523, GenBank AL598959) was cloned in the plasmid vector pAE, which was used for expression of His-tagged recombinant protein in bacterial host E. coli BL21 as described elsewhere (Garay-Malpartida, 2004). We produced the hexamer-histidine-tagged-PIF/DCD protein in E. coli BL21(DE3) by growing transformed bacteria at an absorbance of 0.3-0.4 at 600 nm, in the presence of 1 mM isopropyl-1-thio-β-D-galactopyranoside (Invitrogen) for 4-8 h. The bacterial pellet was sonicated on ice with three 10-s high-intensity pulses in 15 mL of a lysis buffer containing PBS, 10 mM imidazole, 1% Triton X-100, and 1 mM PMSF and then centrifuged at 10,000 g for 30 min. The soluble supernatant was mixed with streptomycin sulfate (3%, w/v) and then centrifuged at 20,000 g for 1 h. The supernatant was then mixed with 250 µL (bed volume) of HisTrap™ resin (Amersham Biosciences) at 4ºC for 12 h. Proteins were eluted with 300-500 µL of elution buffer containing 100 mM imidazole. As a second step in the purification process, aliquots of proteins were applied onto a Sepharose-Q column (Amersham Biosciences) and eluted with 0.1 M Tris-HCl, pH 8.0, and increasing concentrations of NaCl (50-500 mM). At each step, the purity of protein preparations was monitored on 15% SDS-PAGE gel with silver staining using the PlusOne kit (Amersham Biosciences). In all steps, the purity of protein fractions was monitored on 15% SDS-PAGE gels and stained with Coomassie blue or silver staining using PlusOne kit. Mass spectrometry and limited proteolysis analysis The exact molecular masses and amino acid sequences of the purified proteins and trypsin-digest fragments were determined with LC/MS-TOF mass spectrometry (Q-TOF Ultima Micromass, London, UK). For the determination of PIF/DCD tendency to assemble into oligomeric complexes, different solutions of purified protein were prepared in the absence or presence of 10 mM DTT and separated by capillary electrophoresis. Each peak of collected fraction was analyzed by LC/MS-TOF mass spectrometry. The peptide map was obtained after enzymatic digestion with trypsin 1:500 (w/w) for 5, 10 and 30 min of incubation. Individual peptides and mixtures were separated by capillary HPLC coupled to LC/MS-TOF mass spectrometry. The masses of monoisotopic peaks with relative intensity higher than 5% of the most intense peak in the spectrum were used for comparison to a theoretical digestion of the protein by trypsin using the MS-program in the Expasy proteomic server of the Swiss Institute of Bioinformatics. Circular dichroism measurements Circular dichroism (CD) spectra were obtained in aqueous solution, trifluoroethanol (TFE) and micellar and non-micellar sodium dodecyl sulfate (SDS) water mixtures using JASCO J810 spectropolarimeter with Peltier-type temperature controller and a thermostated cell holder, interfaced with a thermostatic bath. The far-UV spectra were recorded in 0.1-cm pathlength quartz cells containing 1 mM PIF/DCD protein in 10 mM Tris-HCl buffer, pH 8.0, at 20ºC. The spectrum is presented as an average of five scans recorded from 190 to 250 nm, at a rate 20 nm/min. The data were corrected for the baseline contribution of the buffer. Crystallization trials We monitored the tendency of the recombinant protein solutions to form aggregates and to grow crystals by vapor diffusion in sitting-drop Cryschem plates using the commercial screen Crystal Screen II from Hampton Research according to the protocol described elsewhere (Scapin et al., 2006). In silico predictions of disorder and order The several online programs were assessed in terms of their ability to predict order and disorder in PIF/DCD primary amino acid sequence. The programs were accessed through the servers: www.pondr.com/, http://globplot.embl.de, www.disprot.org., and www.dis.embl.de. The biophysical properties, posttranslational modifications, patterns, domains, and primary, secondary and tertiary structures in the PIF/DCD protein were assigned online using computational programs at the servers Expasy, NPS®, ELM, DDBASE2, Prosite, Pfam and other open servers. RESULTS In the present study, we investigated the structural and chemical properties of the full-length recombinant PIF/DCD protein expressed in E. coli BL21 (DE3) as fusion protein containing a 6-amino acid His-tag (total 102 amino acid residues). Construction of expression plasmids and dose-dependence studies for the induction and expression of soluble recombinant protein at 1-2 mg per liter of bacterial broth have been described in detail (Garay-Malpartida, 2004). We performed the purification in three steps: Ni2+ affinity, ion exchange and HPLC as described in detail (Majczak, 2005). Purified fractions were separated by SDS-PAGE under reduced conditions and silver stained. The gels revealed an anomalous mobility for the recombinant protein in SDS electropherograms. The observed molecular mass of 16 kDa was 5 kDa higher than the predicted molecular mass of 11 kDa (Figure 1). 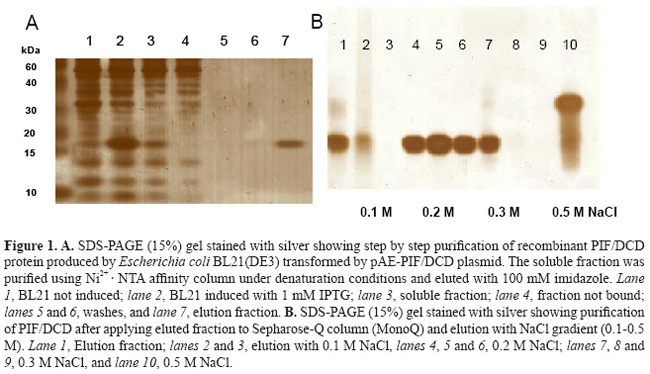
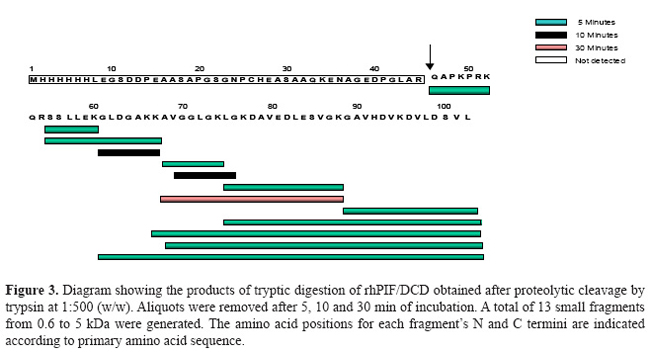
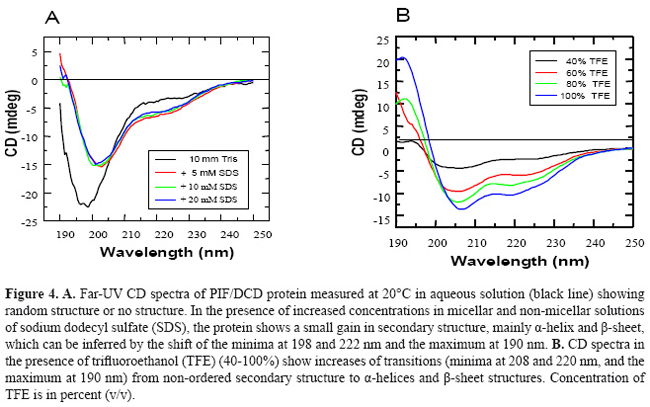
Complementary mass spectrometry (LC/MS-TOF) and oligomerization analysis, under non-reduced conditions, revealed the existence of multiple 21-kDa isoforms (Figure 2B), which we proved to be a dimer formed by two 11-kDa monomer linked by a disulfide bond at cysteine 34 (Majczak, 2005). Proteolytic cleavage of recombinant PIF/DCD protein in the presence of trypsin for 5 min resulted in up to 13 small peptides with molecular masses ranging from 0.6 to 1.1 kDa (Figure 3). A larger peptide of 5 kDa composed of the N-terminal sequence was resistant to trypsin (Figure 3). Finally, we searched in three crystallization trials for a biochemical condition that could lead to stable aggregates and give rise to crystals of PIF/DCD molecules. However, we could not find any folding structures in all conditions tested. Circular dichroism spectra of native PIF/DCD protein recorded at 20ºC, in aqueous solution, indicated that the protein does not exhibit a well-defined secondary structure (Figure 4). The CD spectrum in aqueous solution shows a strong negative peak at 198 nm and small shoulder at 215 nm (Figure 4A, black line) which is indicative of a random coil structure. This CD spectrum was also observed under a wide range of temperatures. However, in the presence of the helicogenic solvent trifluoroethanol (40, 60, 80, 100%) and micellar and non-micellar SDS (5, 10, 20 mM), CD spectra changed completely, indicating that the protein gains α-helical and β-sheet secondary structures.
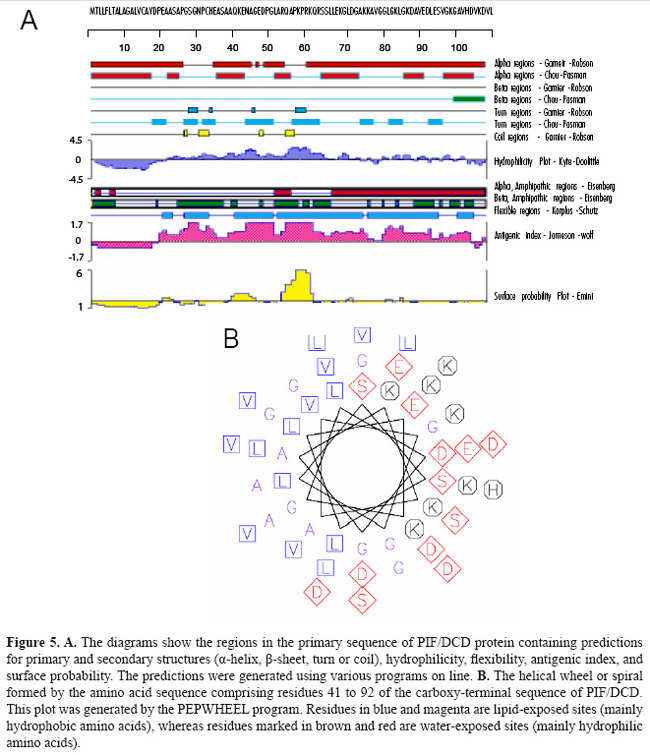
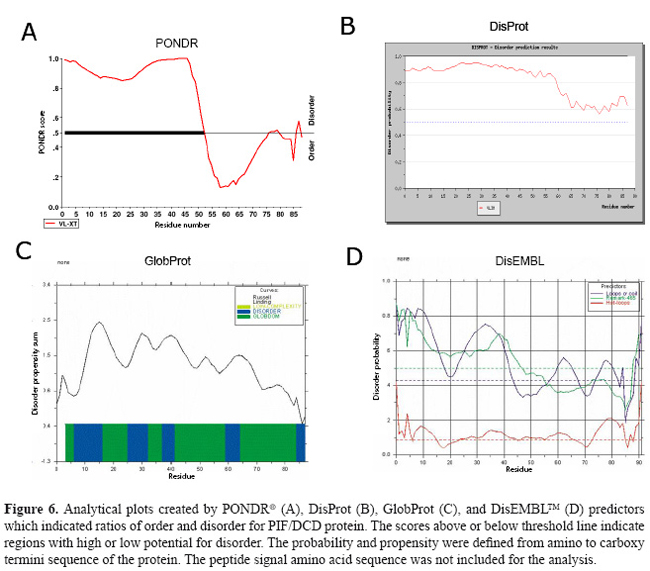
Figure 5 shows the consensus predictions for some biophysical properties, primary and secondary structures identified PIF/DCD protein obtained by a combination of programs. For amino acid sequence, the programs predicted tendencies for α-helix (40%) and random coil (60%) in its structure. The regions comprising the residues 57-70, 79-92 and 93-108 are characterized by imperfect repeats of 12-13 amino acids, which display the variations: KQRSSLLEKGLDGA, KLGKDAVEDLESV, and KGAVHDVKDVLDSVLS, respectively. This amphipathic α-helix is a feature of many peptide antibiotics from animals and plants (Zanetti et al., 1995). The region is formed by cluster of negative/hydrophobic and positive/hydrophilic amino acids that displays a perfect helical wheel, as shown in Figure 5B. Its presence supports the previous study suggesting that the 47-amino acid peptide derived from the C-terminal region of PIF/DCD polypeptide acts as an anionic antibiotic secreted by sweat glands (Schittek et al., 2001). Figure 6 shows the plots created by PONDR (A), DisProt (B), GlobProt (C), and DisEMBL (D) predictors, using the PIF/DCD primary sequence. The curves indicate the ordered regions with globularity propensity, or with high and low potential for high flexibility or intrinsic disorder (Linding et al., 2003a; Romero et al., 2004). The plots show that the sequence comprising the amino acids 1 to 50 is the region with the highest prediction for unstructured region in the putative protein structure. The disorder probability in this region varied from 0.5 to 0.9 (Figure 6). The DisEMBL program characterized the presence of loops or coils (amino acids 1-44, 59-68) and Remark-465 (amino acids 1-47) in this flexible N-terminal segment. Last, we chose an unstructured region, spanning from residues 20 to 45 of the PIF/DCD N-terminus, and a computational method for identifying conformational motions of peptides under many conventional molecular dynamic stimulation conditions. The analysis showed predominantly unfolding trajectories in water over a wide range of temperatures, indicating lack of foldability of the peptide chain (data not shown). Together, these results could explain the absence of crystallized proteins under various biochemical conditions tested.
DISCUSSION PIF/DCD is a 110-amino acid protein that is most highly expressed in sweat glands of the skin (Schittek et al., 2001), as well in breast tissue and in the central nervous system (Porter et al., 2003). The amino acid sequence of 11-kDa PIF/DCD protein does not have significant alignments to known proteins or a particular motif that could assign one specific function. A prior study has provided biological evidence that the protein interacts with specific transmembrane receptors to induce the growth and survival of breast cancer cells (Porter et al., 2003), which suggests that the protein may adopt a stable globular structure. It has also been demonstrated that several truncated peptides generated by limited proteolysis of this protein exert other important biological functions such as antimicrobial activity (Steffen et al., 2006), induction of immune response and neuron survival (Cunningham et al., 1998) and induction of skeletal muscle proteolysis during cancer cachexia (Tisdale, 2004). Such functions have been partly credited to either structural conformation (Cunningham et al., 1998; Steffen et al., 2006) or post-transductional modification of the protein (Tisdale, 2004), which are not yet fully elucidated. In this report, we present further biochemical evidence suggesting that higher susceptibility for proteolytic processing and lack of regular secondary structure may be special features associated with PIF/DCD functions. Our initial biochemical studies on SDS-PAGE gels revealed an anomalous electrophoretic mobility for native reduced recombinant human 11-kDa protein which migrated in the gels as a 16-kDa polypeptide, approximately 40% larger than expected (Figure 1). Aberrant mobility and structural flexibility are caused by PEST domains, which are highly charged regions rich in proline, glutamic acid, aspartic acid, and serine or threonine residues, very often associated with rapid protein degradation (Rogers et al., 1986). In agreement with this, we identified a poor PEST sequence at the N-terminus of the protein. The anomalous electrophoretic behavior is also caused by differential SDS binding to large negative charge on certain segments of the protein leading to the formation of α-helical structures. As demonstrated here (Figure 4) and in previous studies (Lai et al., 2005, Steffen et al., 2006), the native PIF/DCD molecule and DCD-derived peptides DCD-1L and LEK-45 are able to self-associate and form oligomers in the presence of SDS molecules. Globularity and disordered regions can be predicted using computational predictors (Linding et al., 2003a). We identified a large disordered region, spanning from residues 1 to 50 of the N-terminal sequence of PIF/DCD protein (Figure 6). The disorder probability/propensity ranged from 0.5 to 0.9. The accuracy of the predictors (PONDR®, DisEMBL™, Disprot, and GlobPlot™) varies from 70 to 84% (Oldfield et al., 2005; Vucetic et al., 2005). DisEMBL predictor examines residues within loops/coils, Remark-465, and residues with missing coordinates (Linding et al., 2003a). PONDR platform predicts features such as loops/coils and hot loops (Li et al., 1999). GlobPlot predicts order/globularity and disorder based on inter-domains containing linear motifs (Linding et al., 2003b). This structural disorder has also been characterized experimentally in circular dichroism studies (Figure 4). Furthermore, molecular dynamic stimulation studies indicated that residues 20 to 45 display predominantly unfolding trajectories in water over a wide range of temperatures. Correlating with these findings, we could not identify any tendency of PIF/DCD protein to form aggregates and yield crystals. Another important feature identified within the N-terminal region of the PIF/DCD was the resistance to proteolytic digestion by trypsin (Figure 3). Previous studies have shown that PIF/DCD expression and effects in the brain and various tumor types are influenced by oxidative stress, inflammatory cytokines, chemicals, and oncogenic transformation (Cunningham et al., 2002; Wang et al., 2003; Minami et al., 2004; Rieg et al., 2004; Lowrie et al., 2006; Deans et al., 2006). It is quite possible that the native PIF/DCD protein secreted by brain neurons and breast cancer cells binds to its putative transmenbrane receptors, which have not been fully characterized (Porter et al., 2003). Another study has provided evidence that PIF/DCD binds to calreticulin, a ubiquitous calcium-binding protein that has a variety of functions (Cunningham et al., 2000). In addition, it was demonstrated that PIF/DCD is a ligand of MUC1, a transmembrane glycoprotein aberrantly overexpressed by breast carcinomas and other carcinomas (Kufe, 2004). It is interesting that MUC1 co-signals with the ErbB2 and FGFR3 receptor tyrosine kinases, which leads to its diffusion to the nucleus where it interacts and represses p53-mediated transcription (Wei et al., 2007). Thus, alternatively, the growth and survival effects mediated by PIF/DCD could also be associated with its ability to bind MUC1 under certain pathological conditions. Finally, it seems most likely that PIF/DCD acquires a well-defined three-dimensional structure to participate in many cellular functions. Therefore, future studies aiming to precisely define its native and functional structures may advance our understanding of its role in normal and cancer cells. ACKNOWLEDGMENTS We thank Dr. Vitor M.S. Bueno, Carlos E. Winter, Shaker Chuck Farah, and Michel Loos for helping in the experiments and data analysis. Research supported by FAPESP (proc. Nos. 01/01000-7 and 2005/56909-0). REFERENCES Cunningham TJ, Hodge L, Speicher D, Reim D, et al. (1998). Identification of a survival-promoting peptide in medium conditioned by oxidatively stressed cell lines of nervous system origin. J. Neurosci. 18: 7047-7060. Cunningham TJ, Jing H, Wang Y and Hodge L (2000). Calreticulin binding and other biological activities of survival peptide Y-P30 including effects of systemic treatment of rats. Exp. Neurol. 163: 457-468. Cunningham TJ, Jing H, Akerblom I, Morgan R, et al. (2002). Identification of the human cDNA for new survival/evasion peptide (DSEP): studies in vitro and in vivo of overexpression by neural cells. Exp. Neurol. 177: 32-39. Deans DA, Wigmore SJ, Gilmour H, Tisdale MJ, et al. (2006). Expression of the proteolysis-inducing factor core peptide mRNA is upregulated in both tumor and adjacent normal tissue in gastro-oesophageal malignancy. Br. J. Cancer 94: 731-736. Garay-Malpartida HM (2004). Clonagem, expressão e caracterização funcional da proteína hPIF (human proteolysis-inducing factor). Doctoral thesis, Universidade de São Paulo, São Paulo. Iakoucheva LM and Dunker AK (2003). Order, disorder, and flexibility: prediction from protein sequence. Structure 11: 1316-1317. Kufe D (2004). Modulation of the interaction of MUC1 with MUC1 ligands. United States Patent Office. Patent Application 60/519,822. Lai YP, Peng YF, Zuo Y, Li J, et al. (2005). Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem. Biophys. Res. Commun. 328: 243-250. Li XP, Romero PM, Rani M, Dunker AK, et al. (1999). Predicting protein disorder for N-, C-, and internal regions. Genome Informatics 10: 30-40. Linding R, Jensen LJ, Diella F, Bork P, et al. (2003a). Protein disorder prediction: implications for structural proteomics. Structure 11: 1453-1459. Linding R, Russell RB, Neduva V and Gibson TJ (2003b). GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res. 31: 3701-3708. Lowrie AG, Wigmore SJ, Wright DJ, Waddell ID, et al. (2006). Dermcidin expression in hepatic cells improves survival without N-glycosylation, but requires asparagine residues. Br. J. Cancer 94: 1663-1671. Majczak G (2005). Caracterização estrutural da proteína recombinante rhPIF/DCD (proteolysis-inducing factor/dermcidin). Doctoral thesis, Universidade de São Paulo, São Paulo. Markovic J (2003). Expressão gênica do fator indutor de proteólise celular (PIF) e de sua forma variante (PIF-SV) em células normais e malignas. Master’s thesis, Universidade de São Paulo, São Paulo. Minami Y, Uede K, Sagawa K, Kimura A, et al. (2004). Immunohistochemical staining of cutaneous tumours with G-81, a monoclonal antibody to dermcidin. Br. J. Dermatol. 151: 165-169. Oldfield CJ, Ulrich EL, Cheng Y, Dunker AK, et al. (2005). Addressing the intrinsic disorder bottleneck in structural proteomics. Proteins 59: 444-453. Porter D, Weremowicz S, Chin K, Seth P, et al. (2003). A neural survival factor is a candidate oncogene in breast cancer. Proc. Nat. Acad. Sci. USA 100: 10931-10936. Rieg S, Garbe C, Sauer B, Kalbacher H, et al. (2004). Dermcidin is constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. Br. J. Dermatol. 151: 534-539. Rogers S, Wells R and Rechsteiner M (1986). Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234: 364-368. Romero P, Obradovic Z and Dunker AK (2004). Natively disordered proteins: functions and predictions. Appl. Bioinformatics 3: 105-113. Scapin SM, Carneiro FR, Alves AC, Medrano FJ, et al. (2006). The crystal structure of the small GTPase Rab11b reveals critical differences relative to the Rab11a isoform. J. Struct. Biol. 154: 260-268. Schittek B, Hipfel R, Sauer B, Bauer J, et al. (2001). Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2: 1133-1137. Steffen H, Rieg S, Wiedemann I, Kalbacher H, et al. (2006). Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob. Agents Chemother. 50: 2608-2620. Tisdale MJ (2004). Tumor-host interactions. J. Cell. Biochem. 93: 871-877. Todorov P, Cariuk P, McDevitt T, Coles B, et al. (1996). Characterization of a cancer cachectic factor. Nature 379: 739-742. Tompa P (2002). Intrinsically unstructured proteins. Trends Biochem. Sci. 27: 527-533. Vucetic S, Obradovic Z, Vacic V, Radivojac P, et al. (2005). DisProt: a database of protein disorder. Bioinformatics 21: 137-140. Wang Z, Corey E, Hass GM, Higano CS, et al. (2003). Expression of the human cachexia-associated protein (HCAP) in prostate cancer and in a prostate cancer animal model of cachexia. Int. J. Cancer 105: 123-129. Wei X, Xu H and Kufe D (2007). Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res. 67: 1853-1858. Zanetti M, Gennaro R and Romeo D (1995). Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374: 1-5. |
|