ABSTRACT. The cell wall of a human pathogenic fungus is in contact with the host, serves as a barrier against host defense mechanisms and harbors most fungal antigens. In addition, cell wall biosynthesis pathways have been recognized as essential to viability and as specific drug targets. Paracoccidioides brasiliensis is a dimorphic fungus that presents mycelium morphology in the free environment and causes infection in a yeast form. The morphogenetic conversion is correlated with changes in the cell wall composition, organization and structure. Based on transcriptome analysis, the enzymes involved in the biosynthesis and remodeling of cell wall polysaccharides, as well as several cell wall-associated molecules of P. brasiliensis, were identified and addressed in further detail. Key words: Paracoccidioides brasiliensis, Cell wall, Dimorphism, Cell wall-associated enzymes INTRODUCTION The cell wall of a fungal pathogen is in permanent contact with host cells; it acts as a filter and a reservoir for molecules, such as antigens and enzymes, thus also having an active role during infection (Latgé, 1999). The cell wall is apparently a dynamic structure, whose constituent polymers are continuously modified and rearranged during its biosynthesis (Popolo et al., 2001). Paracoccidioides brasiliensis is a dimorphic fungus that causes paracoccidiodomycosis. As with other pathogenic fungi, culture conditions influence the cell wall composition and morphology of P. brasiliensis (Kanetsuna et al., 1969; San-Blas and Vernet, 1977; Da Silva et al., 1994). We examined cell wall metabolism in P. brasiliensis, by searching for genes in its transcriptome that could be involved in the construction and maintenance of cell wall polymers. We also looked for cell wall-associated molecules in this fungus and how they were differentially expressed in mycelial and yeast forms. STRUCTURE AND CELL WALL COMPOSITION OF PARACOCCIDIOIDES BRASILIENSIS In P. brasiliensis, as in other fungi, lipids, chitin, glucans, and proteins are the main constituents of the cell wall in both mycelial and yeast forms. The lipid (5 to 10%) and glucan (36 to 47%) content of the cell wall is similar in the two forms. The yeast form has a larger amount of chitin (37 to 48%) than the mycelial form (7 to 18%). The mycelium has a higher concentration of proteins (24 to 41%) when compared to the yeast cells (7 to 14%) (Kanetsuna et al., 1969). The main polysaccharide of the yeast cell wall is a-glucan, whereas the polysaccharides of the mycelium wall are b-glucan and galactomannan (this latter corresponding to about 6% of the total). The yeast a-glucan also contains small amounts of a-1,3- or a-1,6-glycosidic linkages. On the other hand, the mycelial cell wall b-glucan contains mainly b-1,3-glicosidic linkages, with small amounts of the b-1,6-glicosidic linkages. The degree of polymerization of b-glucan is 30 glucose residues joined through b-1,3-bonds and side branches with b-1,6-linkages (Kanetsuna et al., 1972). Electron microscopy studies have shown that the mycelium has a single-layered cell wall with chitin and b-glucan fibrils, whereas the yeast has three layers (Carbonell and Rodriguez, 1968). The inner surface is chitin with some b-glucan and the outer surface is formed by a-glucan (Carbonell, 1972). Low amounts of galactose and mannose were observed in the cell wall of the mycelium (Kanetsuna et al., 1969), which contained 12 times more disulfide linkages than its yeast counterpart. Although chitosan has not been identified in the cell wall of the yeast or the mycelium phase, the gene encoding chitin deacetylase (cda), the enzyme that converts chitin to chitosan, was found to be over expressed in yeast, which was confirmed by cDNA microarray data (Felipe et al., 2003, 2005). In addition, the fluG gene, which initiates conidiophore development in Aspergillus (Lee and Adams, 1994), was found in the mycelium phase, indicating the existence of conidia (Table 1). 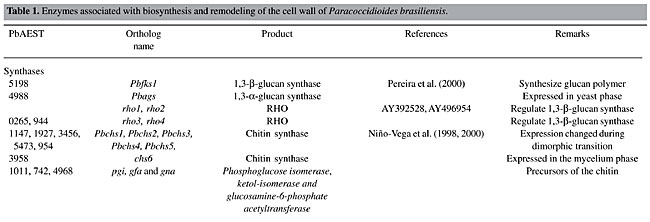 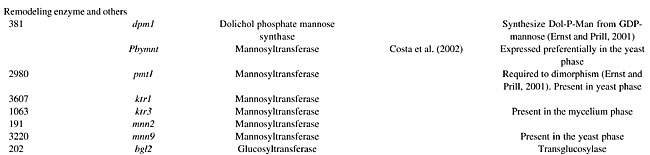   The structure of alkali-extracted water-soluble cell wall polysaccharides (F1SS) from both phases of P. brasiliensis has been studied. The F1SS polysaccharide from mycelium consists of a trisaccharide repeating unit of ®6)-[a-Galf-(1®6)-a-Manp-(1® 2)]-a-Manp-(1®. The F1SS polysaccharides of the yeast phase maintains 10% of the structure of the mycelium phase, but the main structure contains a disaccharide repeating unit of ®6)-[a-Manp-(1®2)]-a-Manp-(1® alternated with a trisaccharide repeating block of ®6)-[b-Galf-(1®6)-a-Manp-(1® 2)]-a-Manp-(1® (Ahrazem et al., 2003). CELL WALL OF PARACOCCIDIOIDES BRASILIENSIS: DIMORPHISM X VIRULENCE The cell wall has an essential role in the pathobiology of P. brasiliensis. The morphogenetic changes are directly associated with the life cycle of this fungus. It undergoes some molecular rearrangement during the morphogenetic switch from hyphae to the yeast phase (Da Silva et al., 1994). Dimorphism has been cited as a crucial factor in the establishment of infection, as strains unable to differentiate into yeast do not cause disease (Borba and Schäffer, 2002). In addition, P. brasiliensis cells presented thicker cell walls after passage in animals than cells subcultured in vitro for many years (San-Blas, 1982), suggesting alterations in the cell wall metabolism. Cell wall polysaccharides, a-1,3-glucan and b-1,3-glucan have been proposed as possible contributors to the dimorphic transition of P. brasiliensis (San Blas and San Blas, 1994). A lower a-1,3-glucan content in the cell wall of the yeast form has been correlated with lower virulence (Hallak et al., 1982). In vitro culture of virulent P. brasiliensis isolates for long periods results in thinner cell walls, loss of virulence and lower a-1,3-glucan levels (San-Blas and San-Blas, 1977). The other main polysaccharide, b-1,3-glucan, has been implicated as an important immunomodulator (Restrepo-Moreno, 1993; Silva et al., 1997). When present together, a- and b-1,3-glucan have been appointed as virulence factors. A hypothesis formulated by Kanetsuna et al. (1972) and modified by San-Blas and San-Blas (1985) explains the differentiation from mycelium to yeast and vice-versa. By the combined activity of b-glucanase and disulfide reductase, the yeast cell wall is loosened around discrete islets of b-glucan, forming a bud. At 37°C the high activity of disulfide reductase, and higher synthesis of chitin and a-glucan than b-glucan result in the yeast form. At 22°C the disulfide reductase has low activity, a-glucan synthesis occurs at low rates and long b-glucan fibrils are formed at the budding sites. IDENTIFICATION OF CELL WALL-ASSOCIATED MOLECULES Classes of cell surface proteins have been described. Proteins released by extraction from intact cells with reducing agents are named cell wall proteins, whereas proteins linked to b-1,3-glucan through a connecting b-1,6-glucan moiety are named GPI-dependent cell wall proteins. The P. brasiliensis transcriptome presents expressed sequencing tags (ESTs) encoding for both kinds (Table 1). Electrostatic forces are involved in the attachment of microorganisms to several types of surfaces (van Oss et al., 1986), and they may be relevant to the interaction between the microorganism and the host cell (Hesketh et al., 1987). Sialic acid residues are constituents of many glycoconjugates and are the major ionogenic compounds that contribute to the negative surface charge of many cell types (Schauer, 1982). Yeast and mycelial forms of P. brasiliensis express surface sialic acid units (Soares et al., 1993). Analysis of the surface anionogenic groups and sialoglycoconjugate structures of yeast forms suggests that sialic acid residues are the main anionogenic groups on the P. brasiliensis surface (Soares et al., 1998). Hydrophobins are small proteins secreted as monomers that self-assemble into an amphipathic film at hydrophilic/hydrophobic interfaces covering fungal structures (Wösten and de Vocht, 2000). Two hydrophobin single-copy genes (Table 1) are present in the P. brasiliensis genome, and Northern blot analysis revealed that both mRNAs are mycelium-specific and highly accumulated during the first 24 h of the mycelium-to-yeast transition (Goldman et al., 2003; Albuquerque et al., 2004). CELL WALL-ASSOCIATED ENZYMES Synthases 1,3-b-glucan synthase In P. brasiliensis, 1,3-b-glucan synthase requires uridine diphosphate glucose (UDPG) as the preferred nucleotide precursor to the in vitro synthesis of b-glucan (San-Blas, 1979). This reaction is inhibited by GTP and other nucleotides (San-Blas and San-Blas, 1986). To date only one homologue, Pbfks1 (Table 1), has been cloned and characterized (Pereira et al., 2000). Pbfks1 has an open reading frame of 5942 bp, interrupted by two putative introns, codifying a predicted protein of 1926 amino acids (212 kDa). The presence of putative regulatory signals suggests a flexible and complex control mechanism for the expression of Pbfks1, as described for the homologous fks1 and fks2 of S. cerevisiae (Mazur et al., 1995). Although the UDPG-binding motif of the 1,3-b-glucan synthase has not been found yet, analogous domains to the UDPG-binding motif of cellulose synthase, delimited by Kelly et al. (1996), were found in PbFKS1. Figure 1 presents the alignment of putative domains found in A. nidulans, Acetobacter xylinum and P. brasiliensis.
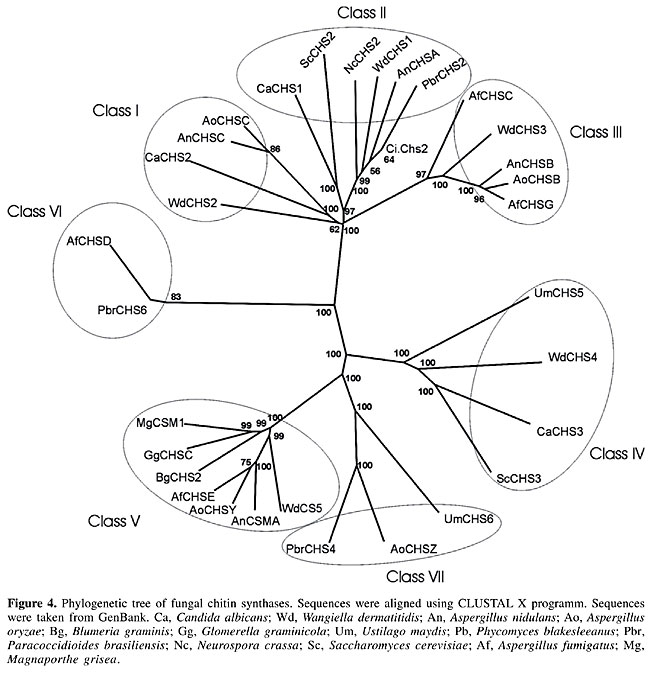
In P. brasiliensis, PbFks1p seems to assemble the phosphorylated glucan polymer and extrude it out of the membrane simultaneously, since the PTS-HPr (phosphotransferase system-phosphoryl carrier protein) phosphorylation site motif was found in the predicted protein PbFks1p. Hydropathy analysis putatively classified PbFks1p as an integral membrane protein displaying a catalytic cytoplasmic domain between two transmembrane regions (Pereira et al., 2000). Analysis of particulate preparations of the P. brasiliensis suggested that 1,3-b-glucan synthase localizes mainly to the cytoplasmic membrane (Sorais-Landaez and San-Blas, 1993). 1,3-b-glucan synthase is regulated by the RHO GTPases, which are multifunctional regulators that interact with numerous proteins (Douglas, 2001). The role of RHO1p in regulating 1,3-b-glucan synthase has been studied in pathogenic fungi such as Candida albicans (Kondoh et al., 1997), Aspergillus fumigatus (Beauvais et al., 2001) and Cryptococcus neoformans (Tanaka et al., 1999). Two rho ESTs, rho3 and rho4, were identified in the transcriptome of P. brasiliensis. Although rho1 (AY392528) and rho2 (AY496954) have already been cloned, their sequences are still partial. In fact, rho1, rho2, rho3, and rho4 present the ATP/GTP-binding site motif ([AG]-x(4)-G-K-[ST]) and residue alignment by the CLUSTAL X program estimated similarity of 26 to 42%, indicating that they are distinct genes (Figure 2).
RHO1p activity is regulated by the activator ROM1p (Ozaki et al., 1996) and by repressor SAC7p (Schmidt et al., 1997). SLA1p, a protein that is important for actin nucleation, is required for the localization of RHO1p (Ayscough et al., 1999). In addition, RHO1p signals to the actin cytoskeleton through Bni1p, the key component of the polarisome, which binds to the barbed ends of actin filaments and nucleates microfilament assembly (Delley and Hall, 1999; Pruyne et al., 2002). All the genes described above were present in the P. brasiliensis transcriptome and are listed in Table 1. 1,3-a-glucan synthase 1,3-a-glucan synthase is the main cell wall neutral polysaccharide of the outer capsule of the pathogenic yeast phase of P. brasiliensis and has been proposed as a virulence factor in this fungus (San-Blas et al., 1977), as well as in Blastomyces dermatitidis (Hogan and Klein, 1994) and Histoplasma capsulatum (Klimpel and Goldman, 1988). We have identified the 1,3-a-glucan synthase gene, Ags, in the P. brasiliensis yeast transcriptome (Table 1). To our knowledge, few 1,3-a-glucan synthase genes have been isolated. Figure 3 shows the alignment of 1,3-a-glucan synthases that presented homology on BLAST analysis (Altschul et al., 1990). Although only a short region has been sequenced, 15% identity and 46% similarity were observed.
Chitin synthase Membrane-bound chitin synthase catalyzes the polymerization of GlcNAc (N-acetyl-b-D-glucosaminidase) from cytosolic UDP-GlcNAc into polysaccharide chains that are extruded to the cell wall (Ruiz-Herrera, 1992; Gooday, 1995). Chitin synthesis in fungi is a complex process (Horiuchi and Takagi, 1999), regulated by multigene families and involved in distinct physiological processes (Cabib, 1991; Gaughran et al., 1994). Although it has been possible to express chs genes in a heterologous host, these transmembrane proteins have not yet been produced as soluble recombinant proteins (Bulawa et al., 1986; Silverman et al., 1988). In P. brasiliensis, five chitin synthases have been identified by PCR amplification of conserved chs gene domains (Niño-Vega et al., 2000), two of which have been isolated, namely Pbrchs2 (GenBank accession No. Y09231) and Pbrchs4 (GenBank accession No. AF107624). The deduced amino acid sequence of CHS2p consists of 1043 residues and is highly homologous to other class II fungal chitin synthases. It presents a highly variable region at the cytosolic amino-terminal region, which may be related to a possible zymogenic nature, and a putative catalytic region close to seven membrane-spanning regions at the carboxyl terminus (Niño-Vega et al., 1998). The gene Pbrchs4 codes for a predicted protein, consisting of 1744 amino acids, with a C-terminal domain homologous to chitin synthases and an N-terminal domain with homology to myosin motor-like domains, although the latter does not present classical signatures (Niño-Vega et al., 2004). Despite the fact that yeast cells contain more chitin than do hyphae, the levels of mRNA for Pbrchs1 (GenBank accession No. AF107622), Pbrchs2, Pbrchs3 (GenBank accession No. AF107623), Pbrchs4 and Pbrchs5 (GenBank accession No. AF107625) were higher in the former (Niño-Vega et al., 2000), suggesting that post-transcriptional regulation of chs gene expression is important for morphogenesis. We identified a new chitin synthase (Pbrchs6), which is present only in the mycelium phase of P. brasiliensis. An update of the phylogenetic tree (Niño-Vega et al., 2004) with all CHS predicted proteins of P. brasiliensis is presented in Figure 4. It was constructed by multiple sequence alignments with the Clustal X program, following the neighbor-joining method (Thompson et al., 1997). Robustness of branches was estimated using 100 boot-strapped replicates. The amino acid sequences were viewed with the TreeView software. The tree shows that PbrCHS6 probably belongs to class VI, since it is branched together with AfCHSD, a chitin synthase of this class (GenBank accession No. U62614) from A. fumigatus (100% bootstrap confidence levels of branches). PbrCHS2 and PbrCHS4 belong to class II and VII, respectively. The chs genes from other fungi were grouped in their respective classes.
Remodeling enzymes Manosyltransferase Some cell wall proteins are glycosylated on serine or threonine amino acids by the addition of mannose residues. Dolichol phosphate mannose synthase (DPM1p) synthesizes Dol-P-Man from GDP-mannose, which is the substrate for protein mannosyltransferases (PMT) (Ernst and Prill, 2001). O-glycosylation in many fungal species is initiated in the endoplasmic reticulum by PMTp, which transfer the first mannose to serine or threonine residues, and is completed by mannosyltransferases (MNTp) in the Golgi compartment by the concerted action of a range of mannosyltransferases, including MNT1p, KTR1p, KTR3p, and MNN1p, which attach further mannose residues to the first O-linked mannose sugar (Gow et al., 1999; Ernst and Prill, 2001). PMTp1 and MNTp1 are critical for cell wall structure, fungal adhesion and virulence (Gozalbo et al., 2004). In addition, Pmt is required for dimorphism of C. albicans (Ernst and Prill, 2001). Although they seem functionally redundant in the elongation of the second and third mannose residues of the O-linked mannan oligosaccharide, Pbymnt1, Pbktr1 and Pbktr3, which are present only in mycelium ESTs, were identified in the transcriptome of P. brasiliensis (Table 1). The low identity observed among them (Figure 5) could be due to the shortness of the sequences obtained.
The MNN9 protein is involved in the synthesis of N-linked outer chain mannan. The mnn9 knockouts exhibit characteristic phenotypes of defects in cell wall biosynthesis and/or assembly (Wills et al., 2000). The dpm1, pmt1, mnn2, and mnn9 genes were identified in the transcriptome of P. brasiliensis (Table 1). While mnn2 was present in both phases the others were exclusive to the yeast phase. Cross-linking of cell wall components Studies of cell wall chemical organization in A. fumigatus, coupled with comparative analysis of S. cerevisiae cell wall data, have shown that 1,3-b-glucan branching and chitin-1,3-b-glucan binding are essential exocellular enzymatic steps in cell wall biosynthesis. Cell wall polymers are linked to form an elastic three-dimensional network that acts as a scaffold for the attachment of macromolecules. The final architecture is responsible for the different morphologies of C. albicans (Klis et al., 2001) and P. brasiliensis (San-Blas et al., 2002). Enzymes involved in the integration of 1,3-b-glucan have been described. The transglycosidases play an active role in cell wall synthesis and fungal morphogenesis (Beauvais and Latgé, 2001). Mutations in gel, phr and epd all result in alteration of polar hyphal growth. The absence of pH-related genes, PHR1p and PHR2p, changes the composition of the cell wall, produces aberrant morphologies in cells and causes growth defects, thus possibly impairing adaptation to ecological niches with different pH (Navarro-García et al., 2001). We have identified the bgl2, gas1, crh1, gel1, gel2, and gel3 transglycosidase genes in the P. brasiliensis transcriptome (Table 1). Hydrolases Hydrolytic enzymes, such as 1,3-b-glucanases and chitinases, may have roles in the morphogenetic events required for the softening of the cell wall structure (Wessels, 1988). In spite of this, fungal 1,3-b-glucanases have been poorly studied. In contrast to other fungal b-1,3-endoglucanases reported in the literature that are exocellular, the cell wall-associated fungal b-1,3-endoglucanase has been identified in A. fumigatus (Mouyna et al., 2002). By comparison, several cell wall-associated and secreted chitinases have been found (Mellor et al., 1994; Hearn et al., 1998). Colman and co-workers have identified 10 genes that show daughter-cell-specific expression (Colman-Lerner et al., 2001). These include the endochitinase cts1 (Kuranda and Robbins, 1991), and the putative glucanases scw11 (Cappellaro et al., 1998) and dse4 (Colman-Lerner et al., 2001). They were also discovered in the P. brasiliensis transcriptome (Table 1), and dse4 was differential for the mycelium phase. N-acetyl-b-D-glucosaminidase (NAG) is defined as a glycosyl hydrolase enzyme that, in concerted action with chitinase, promotes efficient degradation of chitin by microorganisms (Soto-Gil and Zyskind, 1989; Gooday et al., 1992). Our group has cloned and characterized Pbnag1, encoding a NAG of family 20 (Santos et al., 2004). Mycelial and yeast forms of P. brasiliensis were tested for several glycohydrolases through the measurement of their activity to investigate a possible role in the morphogenetic process of the fungus. High levels of b-glucanases, as well as low amounts of a-glucanase, chitinase and maltase were found. Tests for invertase, amylase and lactase were negative. The levels of b-1,3-glucanase were higher in the mycelial form. The shift to the mycelial phase correlated with an increase in the levels of b-1,3-glucanase. The enzyme was present in the cytoplasm, cell wall and culture medium, although enzymatic parameters have been slightly different between extracellular and cell wall-bound enzymes (Flores-Carreón et al., 1979). The chitinase, 1,3-b-endo- and exoglucanase (exg1) genes were identified in the transcriptome of P. brasiliensis. Cell wall precursors Several new therapeutic targets have been described in the literature, as well as in screening assays to identify respective inhibitors. Phosphoglucose isomerase (pgi), glutamine: fructose-6-phosphate amidotransferase (gfa) and glucosamine-6-phosphate acetyltransferase (gna), which catalyze consecutive reactions in cell wall metabolism, are critical to the synthesis of their precursors. Since alteration in precursor levels results in fungal cell wall abnormalities, leading to cell death, these enzymes are potential targets for antifungal drugs (Selitrennikoff and Nakata, 2003). These, and several other genes involved in aminosugar metabolism, were identified in the transcriptome of P. brasiliensis (Table 1). CONCLUDING REMARKS Despite our poor knowledge concerning P. brasiliensis cell wall metabolism, especially concerning the biochemical and genetic control mechanisms, we found genes encoding cell wall-related synthases, as well as remodeling enzymes, among others. In a comparison with the better studied S. cerevisiae cell wall biogenesis, which seems to mobilize about 1200 genes, some of them were also found in the P. brasiliensis transcriptome, such as those encoding mannosyltransferases, glucan synthases and chitin synthases. Genes with specific roles in the cell wall of S. cerevisiae present homologies in the P. brasiliensis transcriptome. This fact suggests similar mechanisms in the construction of the cell walls of P. brasiliensis and S. cerevisiae. Elucidation of the respective biological processes and functional mechanisms regulating the synthesis, organization and environmental interactions of this complex structure is required for a fuller understanding of this fungus. ACKNOWLEDGMENTS Research supported by MCT/CNPq, CNPq, CAPES, FUB, and UFG. We are thankful to Hugo Costa Paes for English revision of this text. REFERENCES Ahrazem, O., Prieto, A., San-Blas, G., Leal, J.A., Jiménez-Barbero, J. and Bernabé, M. (2003). Structural differences between the alkali-extracted water-soluble cell wall polysaccharides from mycelial and yeast phases of the pathogenic dimorphic fungus Paracoccidioides brasiliensis. Glycobiology 13: 743-747. Albuquerque, P., Kyaw, C.M., Saldanha, R.R., Brigido, M.M., Felipe, M.S.S. and Silva-Pereira, I. (2004). Pbhyd1 and Pbhyd2: two mycelium-specific hydrophobin genes from the dimorphic fungus Paracoccidioides brasiliensis. Fungal Genet. Biol. 41: 510-520. Altschul, S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403-410. Ayscough, K.R., Eby, J.J., Lila, T., Dewar, H., Kozminski, K.G. and Drubin, D.G. (1999). Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol. Biol. Cell 10: 1061-1075. Beauvais, A. and Latgé, J.P. (2001). Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Update 4: 38-49. Beauvais, A., Bruneau, J.M., Mol, P.C., Buitrago, M.J., Legrand, R. and Latge, J.P. (2001). Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183: 2273-2279. Borba, C.M. and Schäffer, G.M.V. (2002). Paracoccidioides brasiliensis: virulence and an attempt to induce the dimorphic process with fetal calf serum. Mycosis 45: 174-179. Bulawa, C.E., Slater, M., Cabib, E., Au-Young, J., Sburlati, A., Adair Jr., W.L. and Robbins, P.W. (1986). The Saccharomyces cerevisiae structural gene for chitin synthase is not required for chitin in vivo. Cell 46: 213-225. Cabib, E. (1991). Differential inhibition of chitin synthases 1 and 2 from Saccharomyces cerevisiae by polyoxin D and nikkomycins. Antimicrob. Agents Chemother. 35: 170-173. Cappellaro, C., Mrsa, V. and Tanner, W. (1998). New potential cell wall glucanases of Saccharomyces cerevisiae and their involvement in mating. J. Bacteriol. 180: 5030-5037. Carbonell, L.M. (1972). Ultrastructure of Paracoccidioides brasiliensis in culture. In: Paracoccidioidomycosis. Proceedings of the First Pan American Symposium, PAHO, WHO, pp. 21-28. Carbonell, L.M. and Rodriguez, J. (1968). Mycelial phase of Paracoccidioides brasiliensis and Blastomyces dermatitidis: an electron microscope study. J. Bacteriol. 96: 533-543. Colman-Lerner, A., Chin, T.E. and Brent, R. (2001). Yeast Cbk1 and Mob2 active daughter-specific genetic programs to induce asymmetric cell fates. Cell 107: 739-750. Costa, A.A., Gómez, F.J., Pereira, M., Felipe, M.S.S., Jesuíno, R.S.A., Deep Jr., G.S. and Soares, C.M.A. (2002). Characterization of a gene which encodes a mannosyltransferase homolog of Paracoccidioides brasiliensis. Microb. Infect. 4: 1027-1034. Da Silva, S.P., Felipe, M.S.S., Pereira, M., Azevedo, M.O. and Soares, C.M.A. (1994). Phase transition and stage-specific protein synthesis in the dimorphic fungus Paracoccidioides brasiliensis. Exp. Mycol. 18: 294-299. De Groot, P.W.J., Ruiz, C., Vazquez de Aldana, C.R., Duenas, E., Cid, V.J., Del Rey, F., Rodriguez-Pena, J.M., Pérez, P., Andel, A., Caubin, J., Arroyo, J., Garcia, J.C., Gil, C., Molina, M., Garcia, L.J., Nombela, C. and Klis, F.M. (2001). A genomic approach for the identification and classification of genes involved in cell wall formation and its regulation in Saccharomyces cerevisiae. Comp. Funct. Genomics 2: 124-142. Delley, P.A. and Hall, M.N. (1999). Cell wall stress depolarises cell growth via hyperactivation of RHO1. J. Cell Biol. 147: 163-174. Douglas, C.M. (2001). Fungal b(1,3)-D-glucan synthesis. Med. Mycol. 39: 55-66. Ernst, J.F. and Prill, S.K. (2001). O-glycosylation. Med. Mycol. 39 (Suppl. 1): 67-74. Felipe, M.S., Andrade, R.V., Petrofeza, S.S., Maranhao, A.Q., Torres, F.A., Albuquerque, P., Arraes, F.B., Arruda, M., Azevedo, M.O., Baptista, A.J., Bataus, L.A., Borges, C.L., Campos, E.G., Cruz, M.R., Daher, B.S., Dantas, A., Ferreira, M.A., Ghil, G.V., Jesuino, R.S., Kyaw, C.M., Leitao, L., Martins, C.R., Moraes, L.M., Neves, E.O., Nicola, A.M., Alves, E.S., Parente, J.A., Pereira, M., Pocas-Fonseca, M.J., Resende, R., Ribeiro, B.M., Saldanha, R.R., Santos, S.C., Silva-Pereira, I., Silva, M.A., Silveira, E., Simoes, I.C., Soares, R.B., Souza, D.P., De-Souza, M.T., Andrade, E.V., Xavier, M.A., Veiga, H.P., Venancio, E.J., Carvalho, M.J., Oliveira, A.G., Inoue, M.K., Almeida, N.F., Walter, M.E., Soares, C.M. and Brigido, M.M. (2003). Transcriptome characterization of the dimorphic and pathogenic fungus Paracoccidioides brasiliensis by EST analysis. Yeast 20: 263-271. Felipe, M.S., Andrade, R.V., Arraes, F.B.M., Nicola, A.M., Maranhão, A.Q., Torres, F.A.G., Silva-Pereira, I., Poças-Fonseca, M.J., Campos, E.G., Moraes, L.M.P., Andrade, P.A., Tavares, A.H.F.P., Silva, S.S., Kyaw, C.M., Souza, D.P., PbGenome Network, Pereira, M., Jesuíno, R.S.A., Andrade, E.V., Parente, J.A., Oliveira, G.S., Barbosa, M.S., Martins, N.F., Fachin, A.L., Cardoso, R.S., Passos, G.A.S., Almeida, N.F., Walter, M.E.M.T., Soares, C.M.A., Carvalho, M.J.A. and Brigido, M.M. (2005). Transcriptional profiles of the human pathogenic fungus Paracoccidioides brasiliensis in mycelium and yeast cells. J. Biol. Chem. Published online on May 3, manuscript M500625200. Flores-Carreón, A., Gómez-Villanueva, A. and San Blas, G. (1979). b-1,3-glucanase and dimorphism in Paracoccidioides brasiliensis. Antonie van Leeuwenhoek 45: 265-274. Gaughran, J.P., Lai, M.H., Kirsch, D.R. and Silverman, S. (1994). Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isoenzyme Chs3 in vitro and in vivo. J. Bacteriol. 176: 5857-5860. Goldman, G.H., Marques, E.R., Ribeiro, D.C.D., Bernardes, L.A.S., Quiapin, A.C., Vitorelli, P.M., Savoldi, M., Semighini, C.P., Oliveira, R.C., Nunes, L.R., Travassos, L.R., Puccia, R., Batista, W.L., Ferreira, L.E., Moreira, J.C., Bogossian, A.P., Tekaia, F., Nobrega, M.P., Nobrega, F.G. and Goldman, M.H. (2003). Expressed sequence tag analysis of the human pathogen Paracoccidioides brasiliensis yeast phase: Identification of putative homologues of Candida albicans virulence and pathogenicity genes. Eukaryot. Cell 2: 34-48. Gooday, G.W. (1995). Cell walls. In: The Growing Fungus (Gow, N.A.R. and Gadd, G.M., eds.). Chapman and Hall, London, England, pp. 41-74. Gooday, G.W., Wei-Yun, Z. and O´Donnell, R.W. (1992). What are the roles of chitinases in the growing fungus? FEMS Microbiol. Lett. 100: 387-392. Gow, N.A.R., Bates, S., Brown, J.P., Buurman, E.T., Thompson, L.C. and Westwater, C. (1999). Candida cell wall mannosylation: importance in host-fungus interaction and potential as a target for the development of antifungal drugs. Biochem. Soc. Trans. 27: 512-516. Gozalbo, D., Patricia, R., Villamón, E. and María, L.G. (2004). Candida and candidiase: The cell wall as a potential molecular target for antifungal therapy. Curr. Drug Target Infect. Disord. 4: 117-135. Hallak, J., San-Blas, F. and San-Blas, B. (1982). Isolation and wall analysis of dimorphic mutants of Paracoccidioides brasiliensis. Sabouradia 20: 51-62. Hearn, V.M., Escott, G.M., Glyn, E., Evans, V. and Adams, D.J. (1998). Complex chitinolytic system of Aspergillus fumigatus. Microbios 93: 85-104. Hesketh, L.M., Wyatt, J.E. and Handley, P.S. (1987). Effect of protease on cell surface structure hydrophobicity and adhesion of tufted strains of Streptococcus sanguis biotypes I and II. Microbios 50: 131-145. Hogan, L. and Klein, B. (1994). Altered expression of surface alpha-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 62: 3543-3546. Horiuchi, H. and Takagi, M. (1999). Chitin synthase genes of Aspergillus species. In: Aspergillus fumigatus. Contributions in Microbiology (Brakhage, A.A., Jahn, B. and Schmidt, A., eds.). Vol. 2. Karger, Basel, Switzerland, pp. 193-204. Kanetsuna, F., Carbonell, L.M., Moreno, R.E. and Rodriguez, J. (1969). Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J. Bacteriol. 97: 1036-1041. Kanetsuna, F., Carbonell, L.M., Azuma, I. and Yamamura, Y. (1972). Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J. Bacteriol. 110: 208-218. Kelly, R., Register, E., Hsu, M.J., Kurtz, M. and Nielsen, J. (1996). Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178: 4381-4391. Klimpel, K.R. and Goldman, W.E. (1988). Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect. Immun. 56: 2997-3000. Klis, F.M., De Groot, P. and Hellingwerf, K. (2001). Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39 (Suppl. 1): 1-8. Klis, F.M., Mol, P., Hellingwerf, K. and Brul, S. (2002). Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26: 239-256. Kondoh, O., Tachibana, Y., Ohya, Y., Arisawa, M. and Watanabe, T. (1997). Cloning of the RHO1 gene from Candida albicans and its regulation of b-1,3-glucan synthesis. J. Bacteriol. 179: 7734-7741. Kuranda, M.J. and Robbins, P.W. (1991). Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266: 19758-19767. Latgé, J.P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12: 310-350. Lee, B.N. and Adams, T.H. (1994). The Aspergillus nidulans fluG gene is required for production of an extracellular developmental signal and is related to prokaryotic glutamine synthetase I. Genes Dev. 8: 641-651. Mazur, P., Morin, N., Baginsky, W., El-Sherbeini, M., Clemas, J.A., Nielsen, J.B. and Foor, F. (1995). Differential expression and function of two homologous subunits of yeast 1,3-b-D-glucan synthase. Mol. Cell. Biol. 15: 5671-5681. Mellor, K.J., Nicholas, R.O. and Adams, D.J. (1994). Purification and characterization of chitinase from Candida albicans. FEMS Microbiol. Lett. 119: 111-118. Mouyna, I., Sarfati, J., Recco, P., Fontaine, T., Henrissat, B. and Latge, J.P. (2002). Molecular characterization of a cell wall-associated b(1-3)endoglucanase of Aspergillus fumigatus. Med. Mycol. 40: 455-464. Navarro-García, F., Sánchez, M., Nombela, C. and Pla, J. (2001). Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol. Rev. 25: 245-268. Niño-Vega, G.A., Buurman, E.T., Gooday, G.W., San-Blas, G. and Gow, N.A.R. (1998). Molecular cloning and sequencing of a chitin synthase gene (CHS2) of Paracoccidioides brasiliensis. Yeast 14: 181-187. Niño-Vega, G.A., Munro, C.A., San-Blas, G., Gooday, G.W. and Gow, N.A.R. (2000). Differential expression of chitin synthase genes during temperature-induced dimorphic transition in Paracoccidioides brasiliensis. Med. Mycol. 38: 31-39. Niño-Vega, G.A., Carrero, L. and San-Blas, G. (2004). Isolation of the CHS4 gene of Paracoccidioides brasiliensis and its accommodation in a new class of chitin synthases. Med. Mycol. 42: 51-57. Ozaki, K., Tanaka, K., Imamura, H., Hihara, T., Kameyama, T., Nonaka, H., Hirano, H., Matsuura, Y. and Takai, Y. (1996). Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15: 2196-2207. Pereira, M., Felipe, M.S.S., Brígido, M.M., Soares, C.M.A. and Azevedo, M.O. (2000). Molecular cloning and characterization of a glucan synthase gene from the human pathogenic fungus Paracoccidioides brasiliensis. Yeast 16: 451-462. Popolo, L. and Vai, M. (1999). The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426: 385-400. Popolo, L., Gualtieri, T. and Ragni, E. (2001). The yeast cell-wall salvage pathway. Med. Mycol. 39 (Suppl. 1): 111-121. Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A. and Boone, C. (2002). Role of formins in actin assembly: nucleation and barbed-end association. Science 297: 612-615. Restrepo-Moreno, A. (1993). Paracoccidioidomycosis In: Fungal Infections and Immune Responses (Murphy, J.W., Friedman, H. and Berdinelli, M., eds.). Plenum Press, New York, NY, USA, pp. 251-276. Rodríguez-Peña, J.M., Cid, V.J., Arroyo, J. and Nombela, C. (2000). A novel family of cell wall-related proteins regulated differently during the yeast life cycle. Mol. Cell. Biol. 20: 3245-3255. Ruiz-Herrera, J. (1992). Fungal Cell Wall: Structure, Synthesis and Assembly. CRC Press, Boca Raton, FL, USA. San-Blas, G. (1979). Biosynthesis of glucans by subcellular fractions of Paracoccidioides brasiliensis. Exp. Mycol. 3: 249-258. San-Blas, G. (1982). The cell wall of fungal human pathogens: Its possible role in host-parasite relationships. Mycopathologia 79: 159-184. San-Blas, G. and San-Blas, F. (1977). Paracoccidioides brasiliensis: cell wall structure and virulence. Mycopathologia 62: 77-86. San-Blas, G. and San-Blas, F. (1985). Paracoccidioides brasiliensis. In: Fungal Dimorphism (Szaniszlo, P., ed.). Plenum Press, New York, NY, USA, 93. San-Blas, G. and San-Blas, F. (1986). Effect of nucleotides on glucan synthesis Paracoccidioides brasiliensis. Sabouradia 24: 241-243. San Blas, G. and San Blas, F. (1994). Biochemistry of Paracoccidioides brasiliensis dimorphism. In: Paracoccidioidomycosis (Franco, M., Lacaz, C., Restrepo-Moreno, A. and Del Negro, A., eds.). CRC Press, Boca Raton, FL, USA, pp. 49-66. San-Blas, G. and Vernet, D. (1977). Induction of the synthesis of cell wall a-1,3-glucan in the yeastlike form of Paracoccidioides brasiliensis strain IVIC Pb9 by fetal calf serum. Infect. Immun. 15: 897-902. San-Blas, G., San-Blas, F. and Serrano, L.E. (1977). Host parasite relationships in the yeastlike form of Paracoccidioides brasiliensis strain IVIC Pb9. Infect. Immun. 15: 343-346. San-Blas, G., Nino-Vega, G. and Iturriaga, T. (2002). Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40: 225-242. Santos, M.O., Pereira, M., Felipe, M.S.S., Jesuino, R.S.A., Ulhoa, C.J., Soares, R.B.A. and Soares, C.M.A. (2004). Molecular cloning and characterization of a cDNA encoding the N-acetyl-b-D-glucosaminidase homologue of Paracoccidioides brasiliensis. Med. Mycol. 42: 247-253. Schauer, R. (1982). Chemistry, metabolism and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 40: 131-234. Schmidt, A., Bickle, M., Beck, T. and Hall, M.N. (1997). The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88: 531-542. Selitrennikoff, C.P. and Nakata, M. (2003). New cell wall targets for antifungal drugs. Curr. Opin. Investig. Drugs 4: 200-205. Silva, M.F., Bocca, A.L., Ferracini Jr., R., Figueiredo, F. and Silva, C.L. (1997). Cellular requirements for immunomodulatory effects caused by cell wall components of Paracoccidioides brasiliensis on antibody production. Exp. Immunol. 109: 261-271. Silverman, S.J., Sburlati, A., Slater, M.L. and Cabib, E. (1988). Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 85: 4735-4739. Soares, R.M.A., Alviano, C.S., Angluster, J. and Travassos, L.R. (1993). Identification of sialic acids on the cell surface of hyphae and yeast forms of the human pathogen Paracoccidioides brasiliensis. FEMS Microbiol. Lett. 108: 31-34. Soares, R.M.A., Silva-Filho, F.C., Rozental, S., Angluster, J., Souza, W., Alviano, C.S. and Travassos, L.R. (1998). Anionogenic groups and surface sialoglycoconjugate structures of yeast forms of the human pathogen Paracoccidioides brasiliensis. Microbiology 144: 309-314. Sorais-Landaez, F. and San-Blas, G. (1993). Localization of b-glucan synthetase in membranes of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 31: 421-426. Soto-Gil, R.W. and Zyskind, J.W. (1989). N-N´diacetylchitobiase of Vibrio Harvey. J. Biol. Chem. 264: 14778-14783. Tanaka, K., Nambu, H., Katoh, Y., Kai, M. and Hidaka, Y. (1999). Molecular cloning of homologs of RAS and RHO1 genes from Cryptococcus neoformans. Yeast 15: 1133-1139. Thompson, J.D., Gilbson, T.J., Plewniak, F., Jeanmougin, F. and Higgins, D.G. (1997). The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876-4882. van Oss, C.J., Good, R.J. and Chandhury, M.K. (1986). Surface thermodynamics of bacterial adhesion. J. Coll. Interf. Sci. 111: 378-381. Wessels, J.G.H. (1988). A steady-state model for apical wall growth in fungi. Acta Bot. Neerl. 37: 3-16. Wills, E.A., Redinbo, M.R., Perfect, J.R. and Del Poeta, M. (2000). New potential targets for antifungal development. Emerg. Therap. Targets 4: 1-32. Wösten, H.A. and de Vocht, M.L. (2000). Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta 1469: 79-86. |
|